What will it take for us to see a Practical Cure within the next 15 years? Is it even possible? This report will address both questions.
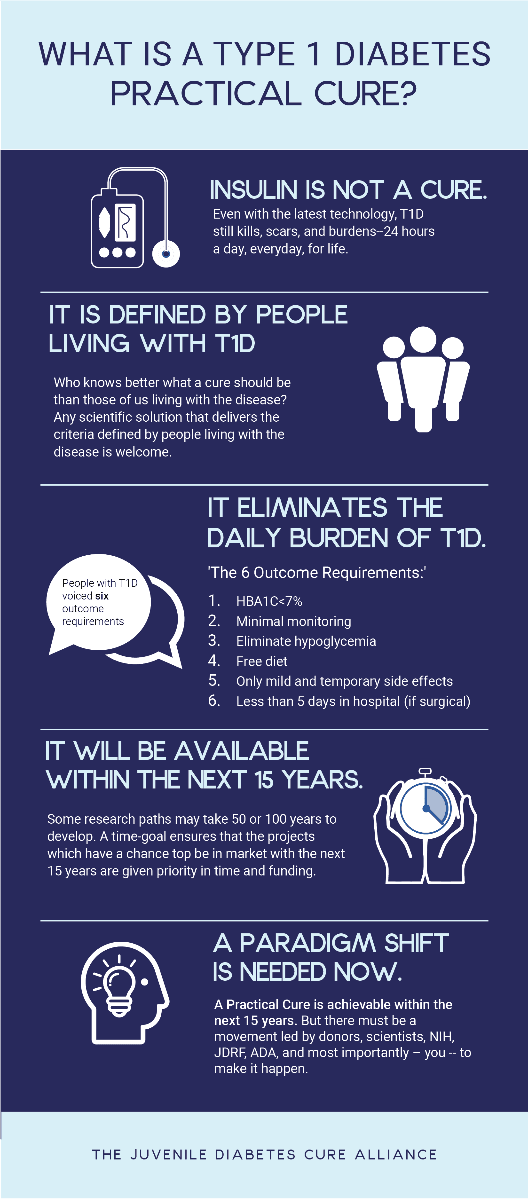
Scientists have pursued a ‘perfect’ T1D cure for nearly 100 years. While that research has led to a better understanding of the disease, it's done little to improve the lives of people with T1D. A Practical Cure may or may not eradicate T1D from the body, but it will eliminate the daily challenges and long-term complications of the disease.
Yesterday the Canadian health regulatory agency gave a landmark approval for a type 1 diabetes human trial that combines stem-cell therapy with gene editing.
This Sunday, November 14 is World Diabetes Day, an event celebrated every year on the birthday of Dr. Fredrick Banting. On this holiday, we acknowledge the significant effort the T1D community puts into advancing a cure every year and reflect on what more we – together – must do to see a cure in our lifetim
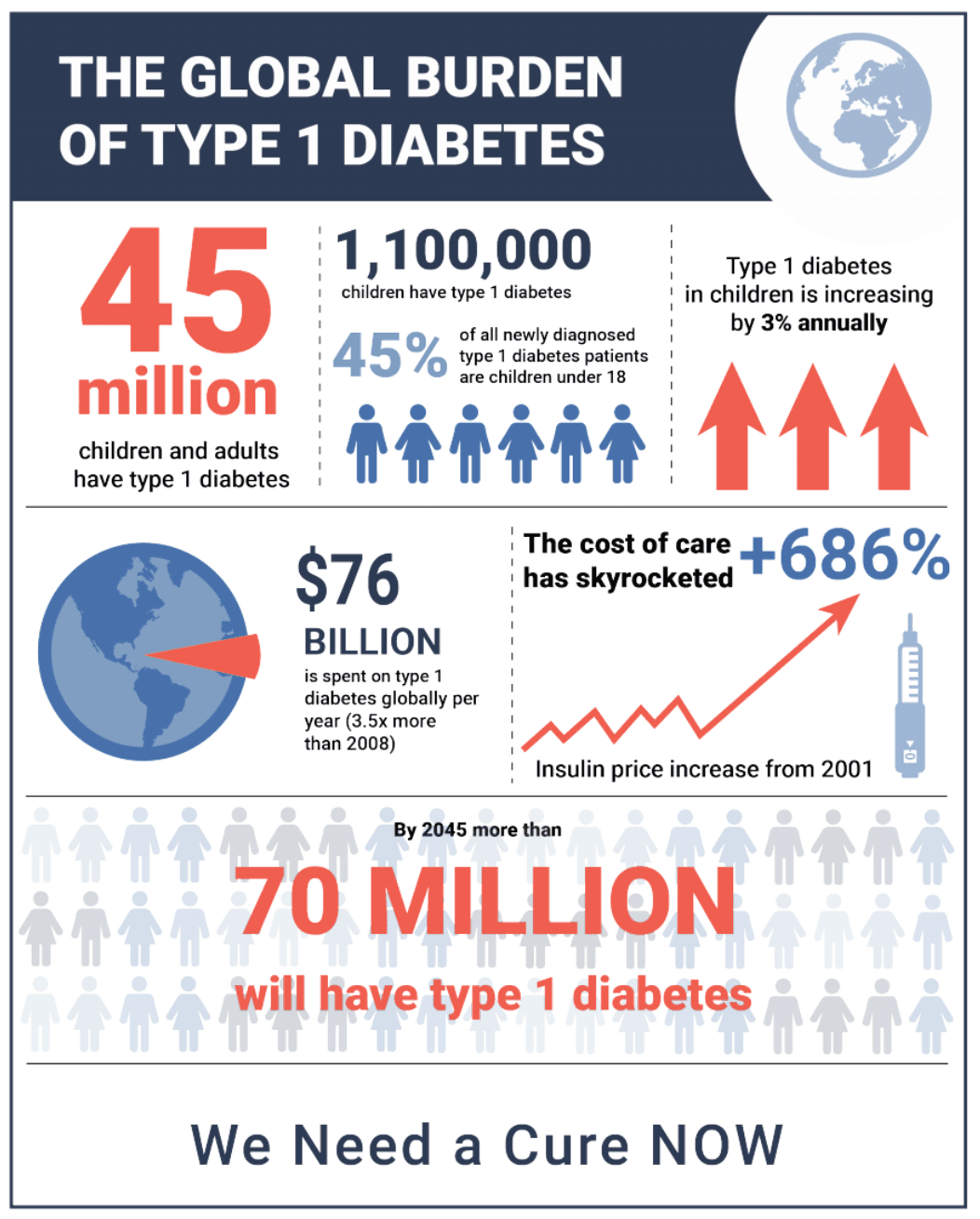
This month, we not only celebrate the resilience of our community, but fight for a future free from the burden of T1D. The infographic identifies an alarming truth: T1D prevalence, particularly in children, is increasing, while the cost of care is skyrocketing.
This special analyst report will evaluate the performance of the JDRF T1D Fund to date. It will assess the Fund's financial decisions, including investments and exits, as well as evaluate the impact the Fund has made to date on progressing a T1D cure.
This morning, Vertex Pharmaceuticals shared an update from its human trial of VX-880, the line of stem-cell-derived insulin-producing beta cells that the company acquired with its purchase of Semma Therapeutics in 2019.
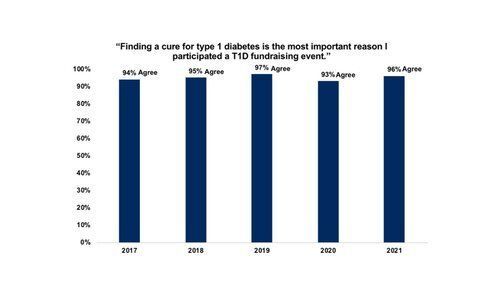
Despite a major shift from in-person to virtual fundraising events since the start of the COVID-19 pandemic, the essential finding of this year’s survey is the same as years before: people donate to and participate in T1D fundraising events because they want to fund cure research.
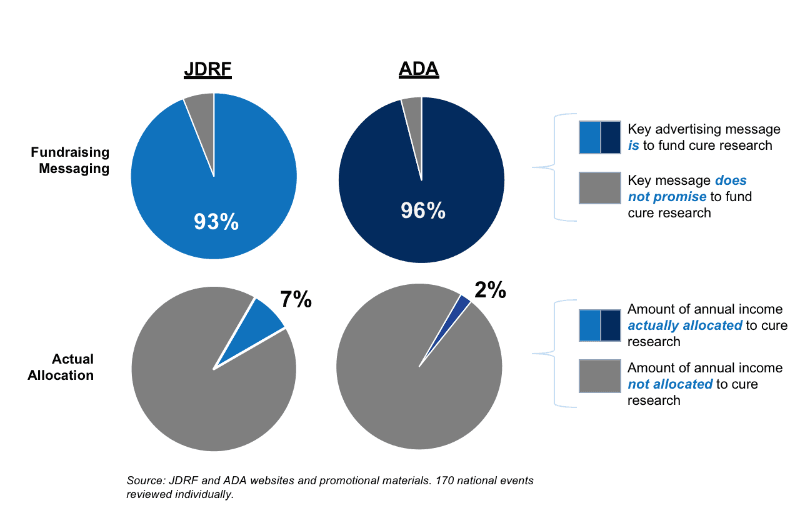
This is the tenth annual review of the messaging used by the ADA and JDRF to advertise national fundraising events. In 2021, the ADA and JDRF advertised a total of 170 (mostly virtual) walks, rides, and galas.
The board of directors is responsible for establishing a non-profit organization’s mission and values, setting strategic direction, overseeing executive leadership, and allocating resources. Ultimately, it is the JDRF and American Diabetes Association (ADA) Boards who decide how to allocate the $300+ million donated to T1D research every year.
Last Friday, The American Diabetes Association (ADA) announced that Chief Executive Officer Tracey D. Brown will step down on October 6, 2021, to take a senior leadership role at Walgreens.
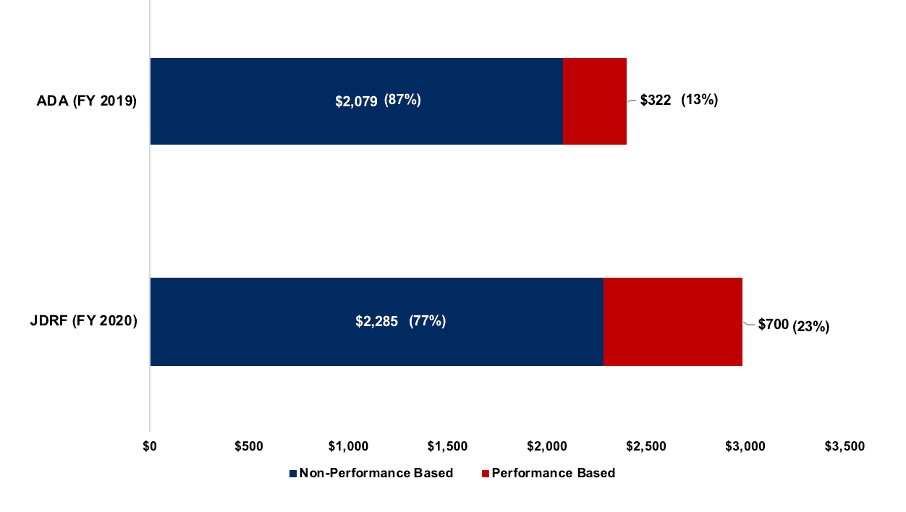
This is the 10th annual review of executive pay at the major T1D non-profits – JDRF and the American Diabetes Association (ADA). Diabetes executives are among the highest-paid in their field, earning more than the top-level executives at similarly sized nonprofits.
Islet cell transplantation (“ICT”) is the only T1D Practical Cure pathway that has shown success in humans to date. This report takes a hard look at where we are today with ICT, where we’ve been, and where we are headed.
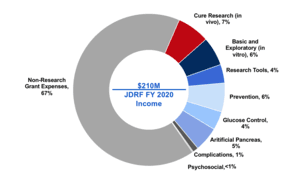
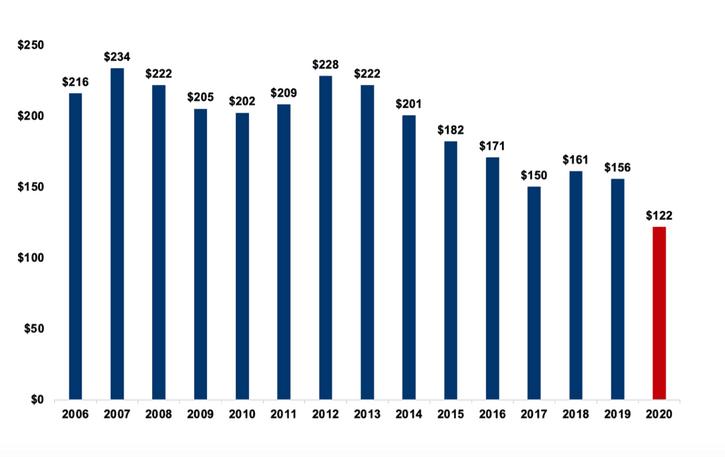
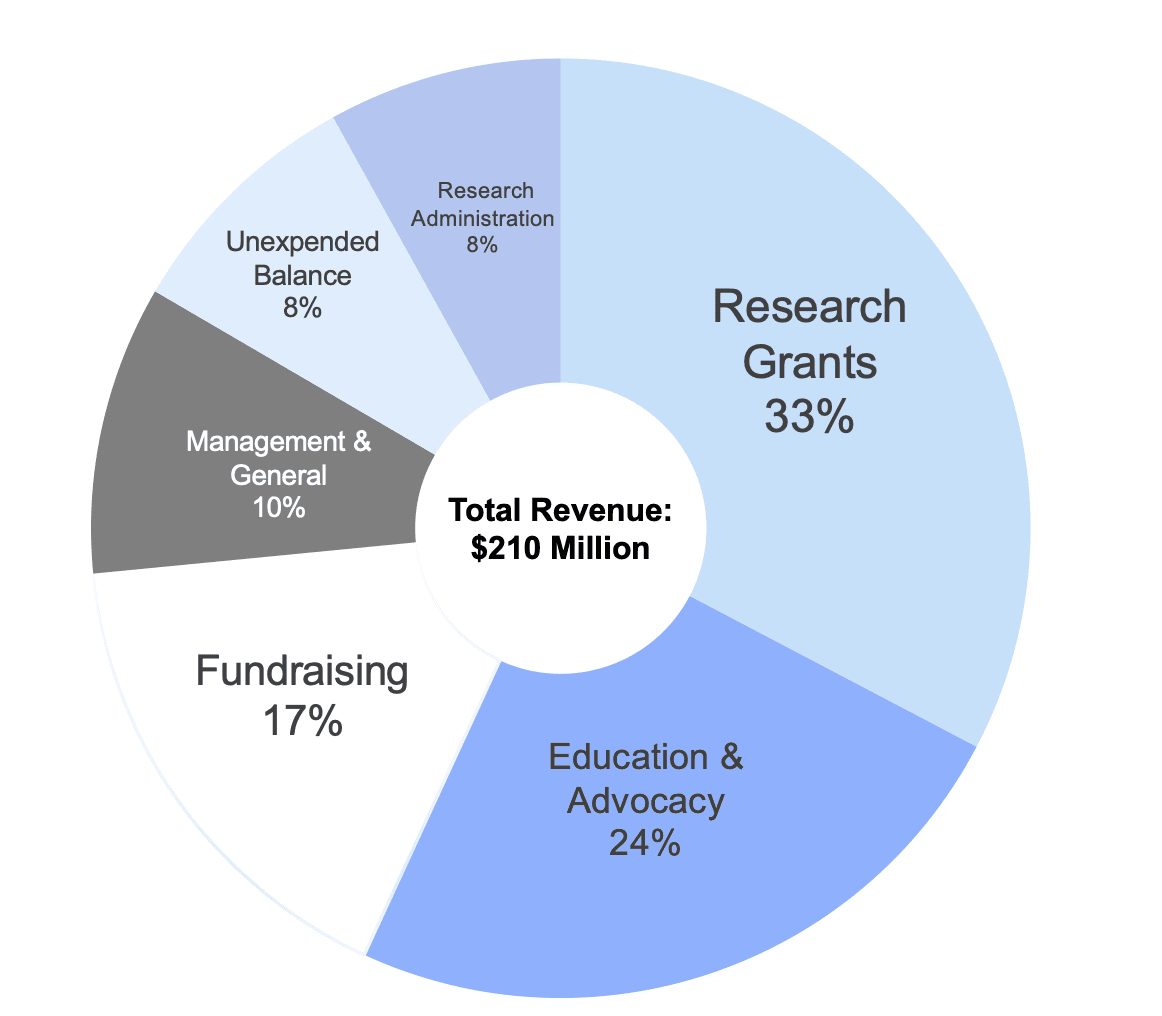
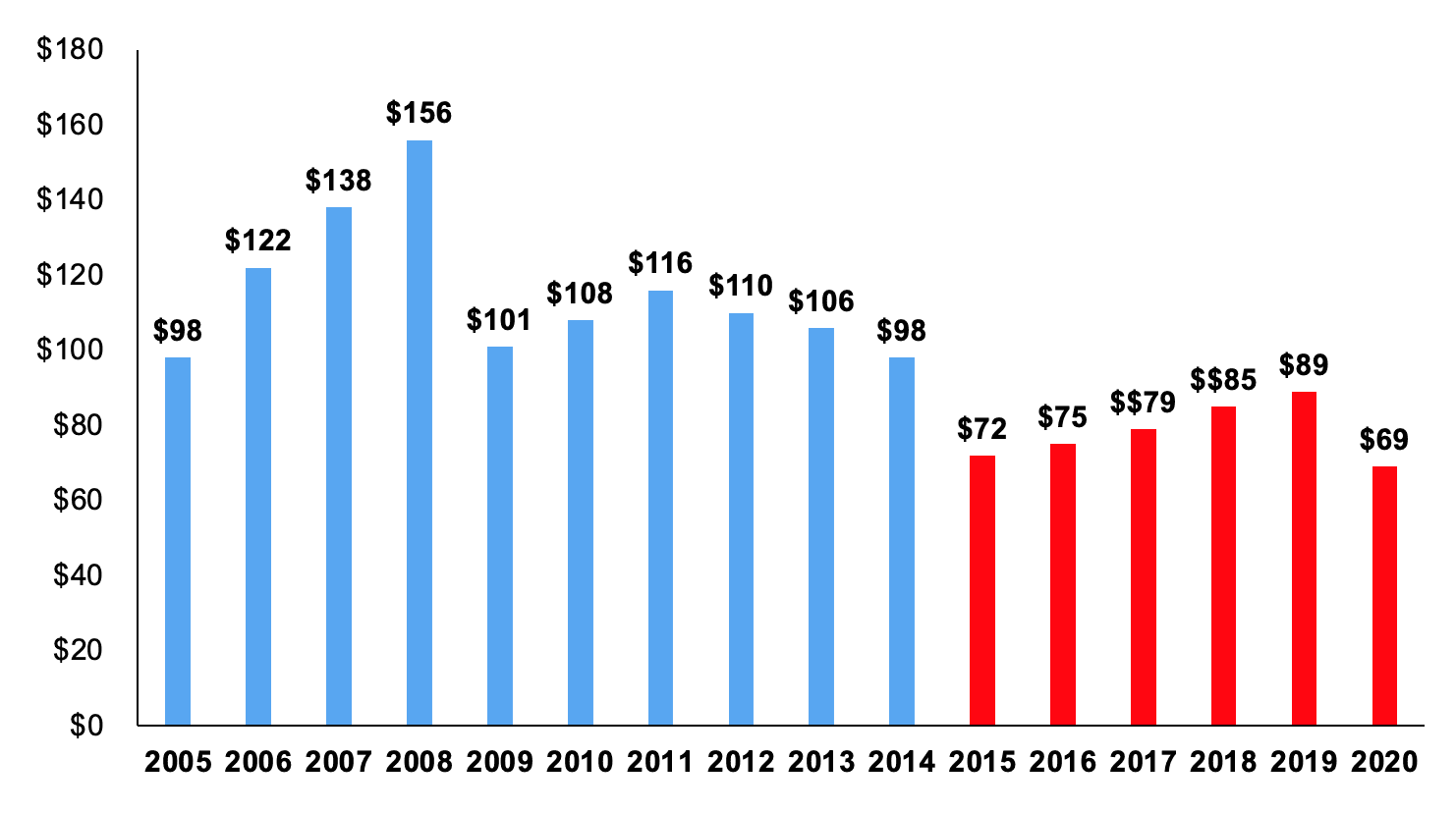
This report analyzes the research grants that JDRF funded in Fiscal Year (FY) 2020. During FY 2020, JDRF spent $69 million of its $210 million revenue (33%) on research grants. This represents a 20-year low in both absolute dollars and as a percentage of total revenue.
Yesterday, Eli Lilly announced that it is acquiring Protomer Technologies, a small biotech company working on a glucose responsive ‘smart’ insulin that could automatically sense rising or dropping sugar levels in the body and activate only when needed.
The American Diabetes Association (ADA) virtually hosted its 81st annual Scientific Sessions last weekend. The conference is one of the year’s most important events for T1D research as it brings top researchers from around the world together to publish, debate, and discuss their latest findings.
Zegalogue, a rescue glucagon made by Zealand Pharmaceuticals, was released today in the United States. This report will review the history of glucagon rescue kits and provide an overview of the options currently available to people with T1D.
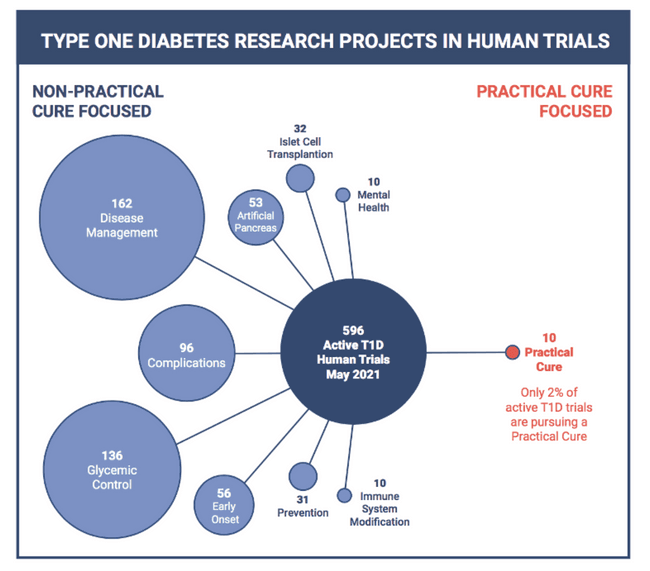
Every medical intervention approved for sale in the United States must first demonstrate it is safe and effective in a series of human trials. Therefore, the T1D trials currently in the registry represent the future of available T1D treatments and cures.
This report will analyze the 2020 financial disclosures of the American Diabetes Association (ADA). With a strong fundraising infrastructure, ties on Capitol Hill, and access to researchers throughout the world, the ADA is well-positioned to act as a forceful lever for T1D cure research. However, the financial documents paint a picture of an organization in turmoil.
On Thursday evening, an FDA advisory committee recommended the approval of teplizumab, an immune-modulating drug that has shown some potential to delay the onset of type 1 diabetes.
This is the third report in a series examining JDRF financial disclosures for Fiscal Year (FY) 2020. It focuses on the costs directly related to the critical task of distributing grants to fund T1D research.
This morning’s Diabetes: Grand Challenge Panel at the World Medical Innovation Forum was a unique event for the T1D community. Three well-known competitors developing stem cell solutions for T1D – ViaCyte, Vertex, and Sigilon – shared the stage and reported on their progress.
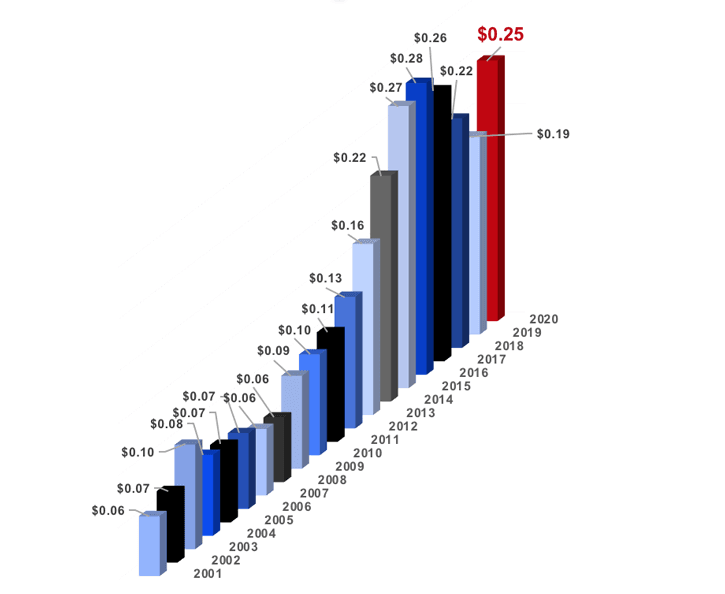
Each year, T1D donors contribute nearly half a billion dollars to the major T1D research organizations: JDRF, the American Diabetes Association (ADA), academic research centers, and others. For over a decade, the JDCA has conducted annual surveys to gauge the priorities, perspectives, and behaviors of these donors.
This report shows how JDRF utilized the money it raised – almost entirely from donor generosity – during Fiscal Year (FY) 2020. Financial statements are where ‘the rubber meets the road’ for nonprofits. The documents show the priorities of the organization and how efficiently it operates.
Last Thursday and Friday, the University of Toronto hosted a virtual research conference that featured some of the world’s most prominent diabetes investigators. This report summarizes the essential points discussed at the conference related to a type 1 diabetes cure.
This morning JDRF released its most recent financial statements for Fiscal Year (FY) 2020, covering the time period from July 1, 2019 through June 30, 2020. This report will provide a quick summary of the main highlights; we will take a deeper look at the financials in the upcoming weeks.
Last weekend, a major diabetes conference brought endocrinologists from around the world together to share the latest research in the field of hormone science. The virtual ENDO 2021 conference was hosted by the Endocrine Society, a professional group of scientists and physicians. This report will highlight a few key topics covered at the conference related to T1D.
New capabilities in gene editing make it relatively easy for scientists to directly edit DNA, the most fundamental building block of life. Although our understanding of what gene editing can do is still in its infancy, current technology offers hope and potential to cure many diseases once thought incurable, including T1D.
This morning, Vertex Pharmaceuticals made two big announcements regarding its T1D cure research program: 1) The FDA gave the research a ‘Fast-track’ designation; and 2) The company officially started its first human trial of VX-880, a line of insulin-producing pancreatic cells derived from stem cells.
Researchers have cured T1D in mice many times over the years. When they do, their results are often shared with great fanfare in press releases that suggest they have cracked the code of a T1D cure. However, none of the successes curing T1D in mice have translated into a cure for humans. This report seeks to answer the following question: What does the next cure in mice mean for us in the T1D community?
The Diabetes Research Institute (DRI) at the University of Miami School of Medicine holds a distinctive position among diabetes research centers. This report provides an overview of the T1D research currently being conducted at DRI.
This morning, ViaCyte announced that it is starting a new Practical Cure (PC) trial testing a new device that is designed to better support its encapsulated cells’ ability to produce insulin in patients with T1D.
Last December, City of Hope, a Southern California cancer research center, began recruiting patients in a trial testing a potential Practical Cure for T1D. This is the first Practical Cure trial funded by the Wanek Family Foundation’s $50 million gift to City of Hope in 2016, which set an ambitious goal to cure T1D within six years.
This is the ninth annual addition of this report. It summarizes progress made during 2020 toward a Practical Cure for type 1 diabetes.