A Phase I study has resulted in some very hopeful headlines about a possible cure. Unfortunately, the headlines are far more promising and hopeful than the actual facts.
This report will provide an overview of the TypeOneNation Summit regarding both technological advances and research.
The board of directors at JDRF and the ADA ultimately have more influence over spending and strategy than anyone else at the organization. Board members set the organization’s strategic direction and have the final say in funding allocations.
Last Friday, the JDCA accepted an invitation to a breakfast meeting in New York with JDRF CEO, Derek Rapp, and John Brady, Chairman of the JDRF International Board, to discuss current and future JDRF priorities.
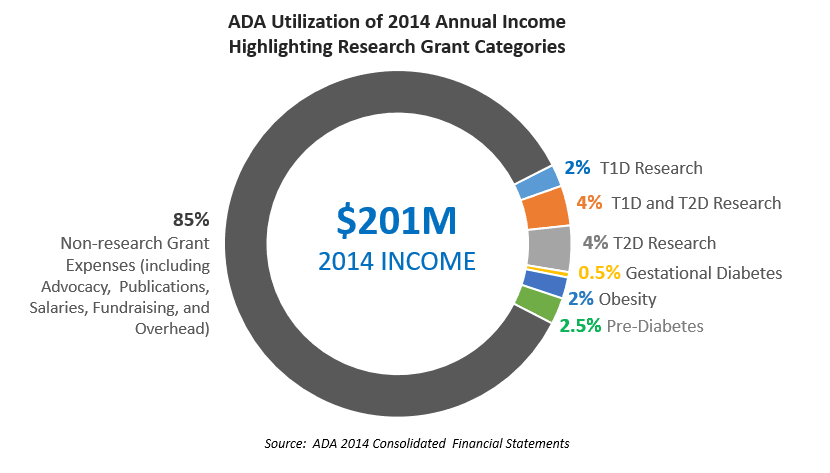
This is the third annual analysis of the ADA's funding for research to help ensure that the priorities of the T1D community are not lost in the ADA's heavy focus on type 2 diabetes.
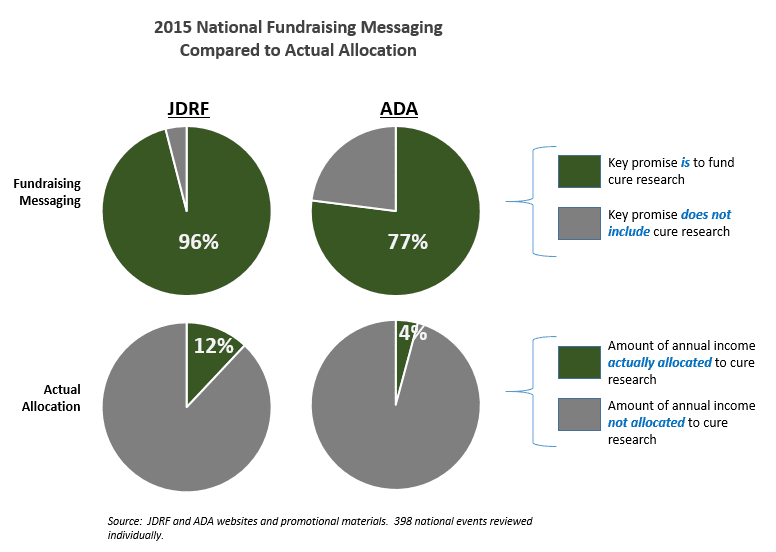
This is the forth annual review of advertising messages used by the ADA and JDRF at national fundraising events.
Starting on October 5th, Dr. Peter Amenta will become the new Joslin President and CEO.
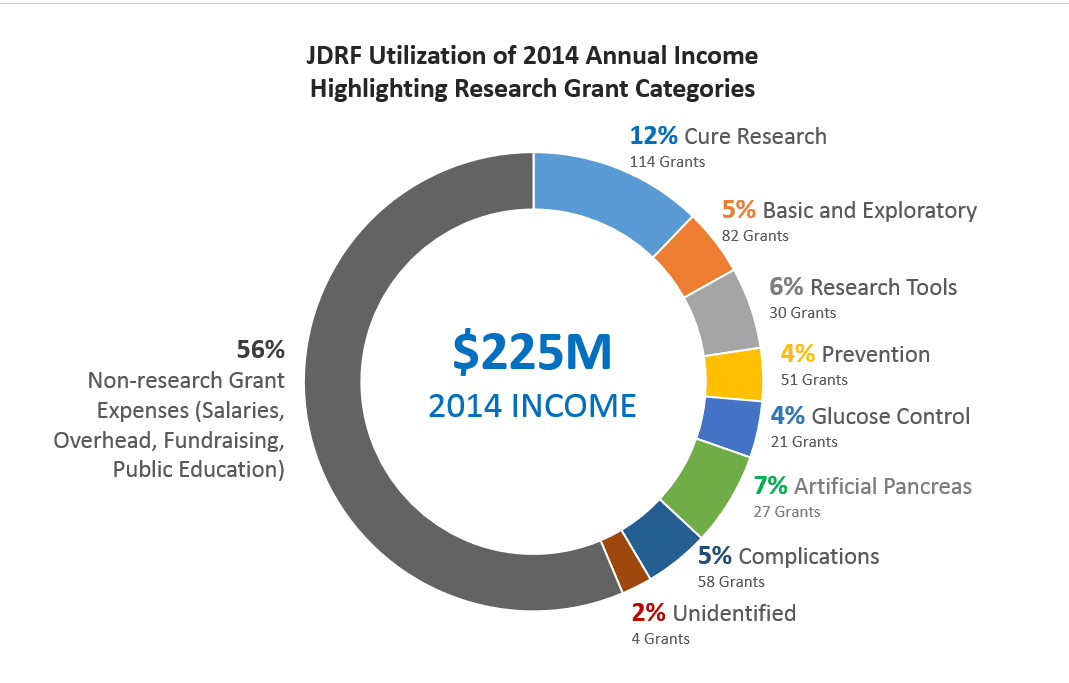
This is the third annual analysis of JDRF's research funding. The main purpose in reviewing and sharing this data is to help ensure that T1D donor and community priorities are being addressed.
The Joslin Diabetes Center released it's CEO of 4.5 years on Monday.
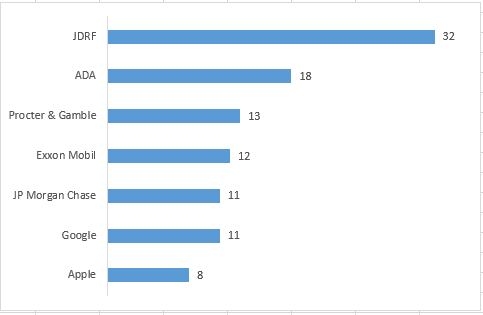
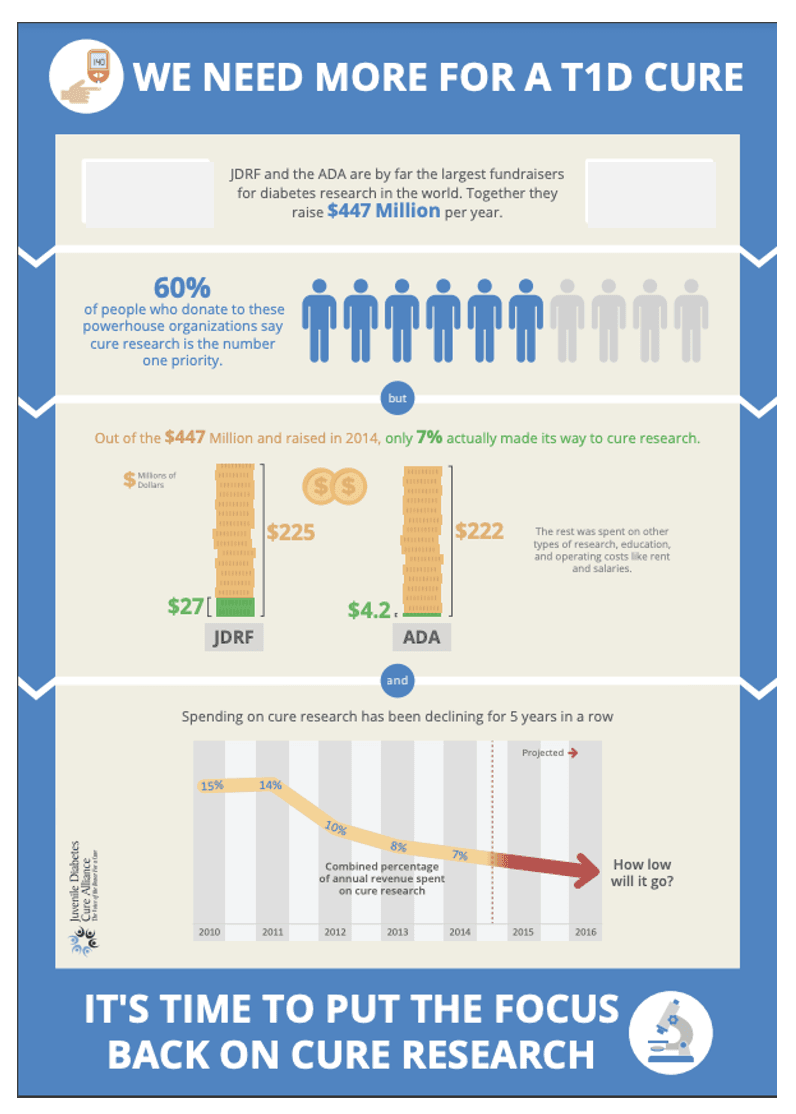
The infographic posted above provides a snapshot of how the ADA and JDRF use their funds.
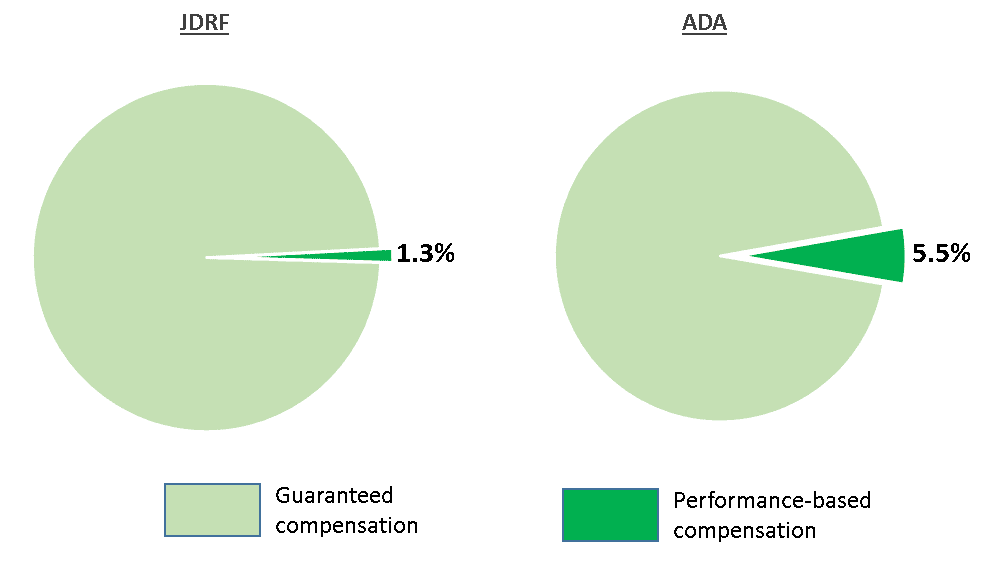
This is the JDCA's 4th Annual Review of executive compensation.
Among the the many artificial pancreas (AP) designs under development, the Bionic Pancreas project led by Dr. Ed Damiano of Boston University is progressing rapidly through human trials. Last week the JDCA had the opportunity to meet face-to-face with Dr. Damiano.
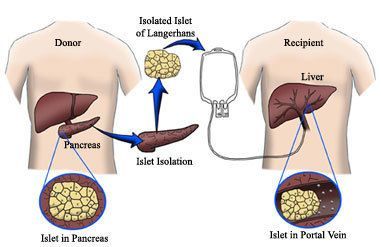
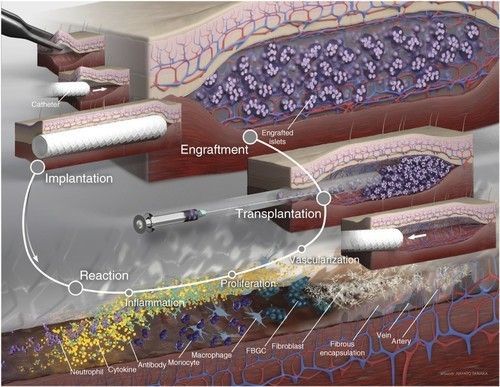
This report provides a basic overview of the status of islet transplantation, one of the four Practical Cure pathways. The JDCA will publish an overview of each of the other three Practical Cure pathways over the coming months.
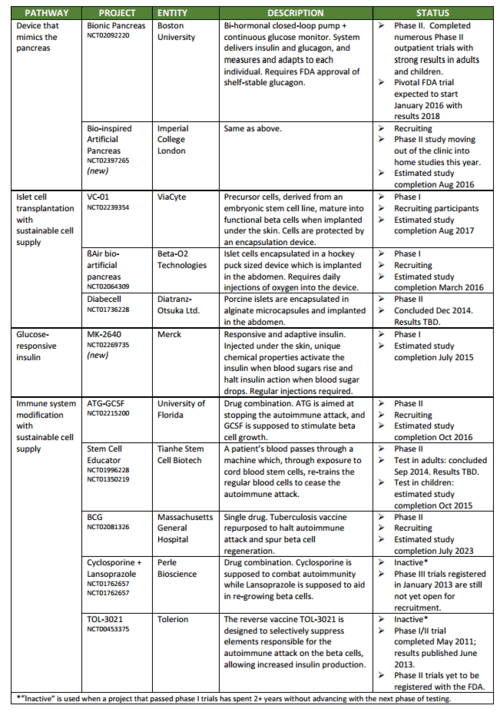
A few weeks ago the JDCA published a report identifying research projects in human trials that may result in a Practical Cure. We also track Practical Cure projects which may begin human trials in the next 24 months. This report provides a list of those projects.
This is the semi-annual review of T1D research in human clinical trials. The review identified 361 open trials that focus on T1D and only 11 initiatives have the potential to deliver a Practical Cure.
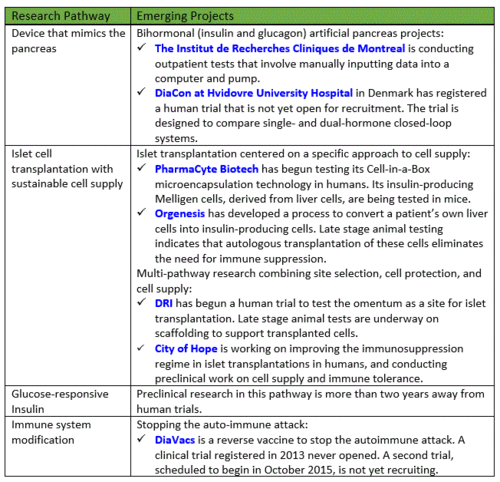
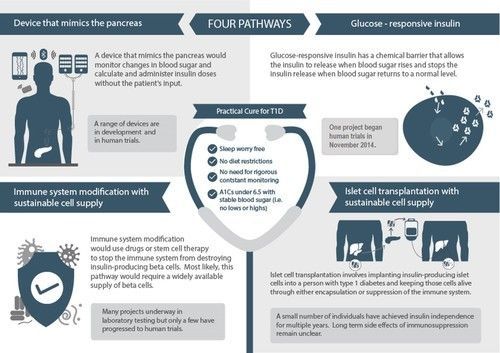
This report is the first in a series of updates on developments in Practical Cure research platforms and projects. It outlines the four research pathways that we believe have the potential to deliver a Practical Cure within the next 15 years.
The Journal of Nature Biology recently published results that appear to be a small step forward in islet transplantation.
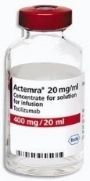
Last week, Bloomberg News reported on a Phase II clinical trial that is testing whether an FDA-approved rheumatoid arthritis drug can be re-purposed to delay or halt the onset of type 1 diabetes.
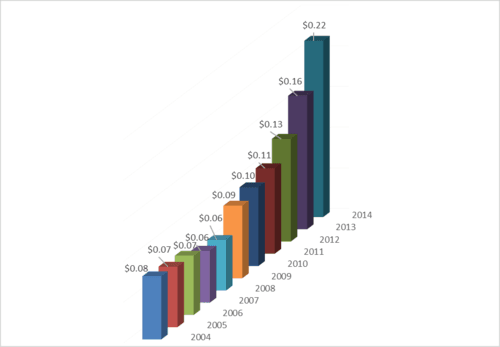
JDRF is becoming less efficient at administering research grants. In 2014, it cost JDRF $.22 to administer each grant dollar of research, the highest level of any year in the past decade.
A new study released by GlobalData, a London-based market research and analytics firm, estimates that the global market for type 1 diabetes treatments will double in the next decade, from $6.6 Billion in 2013 to $13.6 Billion by 2023.
Today, the Boston Globe announced that a new company has been founded to commercialize T1D research out of Dr. Doug Melton’s laboratory at Harvard University.
This is the JDCA's third annual survey-based report on the attitudes and desires of the type 1 diabetes community.