Among the many artificial pancreas (AP) designs under development, the Bionic Pancreas project led by Dr. Ed Damiano of Boston University is progressing rapidly through human trials. Last week the JDCA had the opportunity to meet face-to-face with Dr. Damiano.
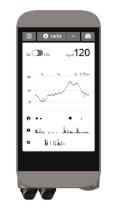
The Bionic Pancreas is a closed-loop dual-hormone artificial pancreas, meaning it administers insulin and glucagon without input from the wearer. A CGM sends data to a pump in which an algorithm calculates and administers doses of insulin and glucagon to keep blood sugar in optimal range.
Unlike other projects we track, the Bionic Pancreas has been tested in adults and children in numerous studies with very strong results. In multi-day trials that have taken place outside a hospital, the Bionic Pancreas consistently stabilizes blood sugars while participants eat as they choose, exercise at varied intensity, and sleep through the night. Trial participants have referred to their experience as life-changing.
Assuming continued strong results through the remainder of this year, the device could be ready to start its final FDA trial in January 2017 at research centers across the U.S.
The main concern about the device is the prolonged use of stable glucagon, which is currently in human testing but not yet approved by the FDA. The effects of daily long-term glucagon use are unknown. Xeris Pharmaceuticals is developing a product that, if successful, could be fast-tracked to the target timetable of the Bionic Pancreas.
To learn more:
Ed Damiano’s TED talk
The Bionic Pancreas Official Website
Joshua Levy's comprehensive overview of the AP landscape
