At a Glance
- Stem cells give rise to all cells in the human body.
- There are three key types: embryonic, adult, and induced pluripotent.
- Scientists began exploring stem cells as a treatment/cure for T1D in the early 2000s.
- In T1D, stem cells can regenerate new insulin-producing pancreatic cells or protect existing ones.
- Several companies and researchers are investigating a stem cell cure for T1D. Vertex Pharmaceuticals is furthest along, with four active human trials.
June 13, 2024
Over the past decade, stem cells have become an increasingly hot topic in medicine. Because stem cells form the basis of our genetics, scientists have been able to manipulate and use stem cells to treat individuals with diseases previously deemed incurable. For instance, Novartis’ drug Kymriah treats and can even cure aggressive forms of blood cancer, Vertex’s drug Casgevy can eliminate the need for blood transfusions in patients with beta-thalassemia, and Allocord's umbilical cord-derived stem cells can treat the rarest genetic disorders.
On the other hand, the use of stem cells has also garnered negative attention, branded as ‘unethical,’ because certain stem cells are sourced from blastocysts, the stage before a cell develops into an embryo.
This report will address stem cell benefits and challenges, focusing on applications toward a cure for T1D.
What Is a Stem Cell?
A stem cell is the building block of all human life. It is a ‘blank’ or partially developed cell that can transform into other cells in the human body: lung, brain, skin, and liver cells, among others. There are three key types of stem cells, and we will go over their origins and developmental potential, particularly regarding T1D.
What Are the Types of Stem Cells?
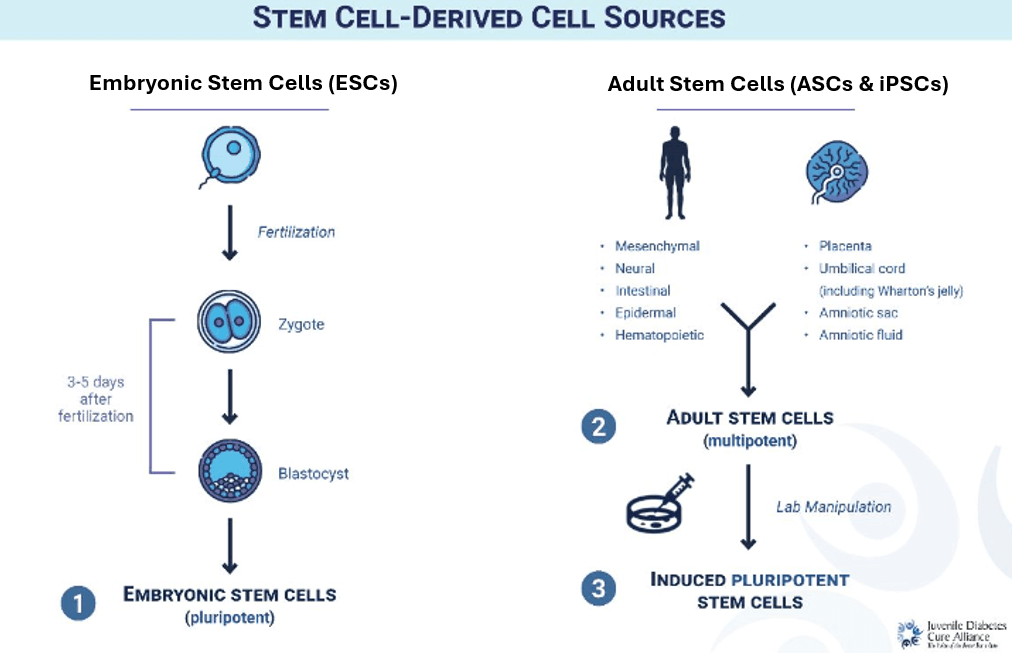
Three main types of stem cells are defined below (see "Stem Cell-Derived Cell Sources").
- Embryonic stem cells (ESCs) come from human zygotes 3 to 5 days after fertilization in vitro (in a lab) and are in the blastocyst stage. These cells are in the most undeveloped state—they are ‘unmapped’—and can become any type of cell. Donated zygotes are initially hard to find due to a lack of donors; however, once obtained, just one zygote can yield an indefinite amount of ESCs.
- Adult stem cells (ASCs) refer to stem cells from two sources: adult tissue and the membranes outside of an embryo (placenta, umbilical cord, and amniotic fluid/sac). In adults, these stem cells are found in specific regions such as bone marrow and skin. As a part of specific organ systems, they are more developed and can only become certain types of cells. ASCs are much easier to obtain than ESCs but cannot be multiplied indefinitely.
- Induced pluripotent stem cells (iPSCs) are adult stem cells that have been harvested and reprogrammed into an unmapped state with the ability to become any type of cell, like ESCs. This allows scientists to easily and ethically source stem cells with indefinite potential. However, the process of creating iPSCs is both lengthy and expensive.
History and Ethics of Stem Cells
Stem cells were first discovered in humans in the early 1960s while scientists researched blood cell formation. In 1963, the first ever stem cell transplant was performed on a man with a terminal form of blood cancer—curing him. Since then, scientists have made strides in learning how to extract, grow, and use stem cells to treat blood disorders, osteoarthritis, spinal cord injuries, corneal damage, neurodegenerative diseases like Parkinson’s, autoimmune diseases, severe burns/wounds, and others.
Ethical and political concerns have been raised over the years, casting doubt on the overall viability of using all types of stem cells. However, concerns center on only one of three stem cell sources: ESCs. The process of obtaining ESCs entails collecting the undeveloped stem cells of a blastocyst, the stage before these cells form an embryo. Some believe this is “unethical” because of the cells’ potential to become a human life. The US federal government passed a law in 1996 limiting the federal funding of research using ESCs. Since then, the law has been revisited and amended several times, though many restrictions remain.
Stem cell proponents advocate for the stigma behind stem cells to be removed, as the cells are lab-grown and gathered before any real development has taken place, and equating it to a person inhibits advancements in cure research. To circumvent this issue, some advocate for increased access, exploration, and use of iPSCs as they have the same capabilities as ESCs without controversy.
In recent years, stem cell research has surged despite ongoing ethical controversy. Research has shown that stem cells can completely change the paradigm in regenerative medicine, a field dedicated to developing technologies to repair cells/tissues damaged by disease or injury to restore function. Likewise, ESCs and iPSCs can be programmed into specific cells to repair, regenerate, and replenish damaged cells. Therefore, stem cells have the potential to cure degenerative diseases that currently are only symptomatically treated and managed—including T1D.
Stem Cells and T1D
Scientists first began exploring the use of stem cells in treating and possibly curing T1D in 2007. In their most unmapped state, stem cells can be programmed into insulin-producing cells and given to people with T1D, potentially restoring their ability to produce insulin.
A stem cell treatment must be accessible, affordable, mass-produced, and protected from the inevitable immune system attack. Several researchers and biomedical companies have begun their pursuits in finding a stem cell cure–including cell supply and cell protection–each with a different approach. A few of these research projects have started human trials (see Appendix A).
What Can T1D Expect from Stem Cell Research?
Stem cells demonstrate the potential to become a Practical Cure for T1D. Each of the three types of stem cells presents us with a unique avenue to combat the cell supply issue T1D researchers face today. Once a stem cell line is initially procured, iPSCs and ESCs can be replicated indefinitely, boosting T1D research capabilities typically hindered without a steady supply of cells. A greater supply of cells allows for greater cell protection research to be conducted, which is necessary to solve the largest hurdle in T1D cure research today: the immunosuppressive barrier.
Several companies are working with stem cells in efforts to achieve a T1D Practical Cure, with more sure to appear in the time ahead. Of those currently working with stem cells, only one company, Vertex, is in human trials. Nevertheless, there is still a long road ahead in finding a stem cell Practical Cure as we await the results of the companies currently conducting research.
JDCA will keep you updated on these results and the ongoing pursuits to find a stem cell cure for T1D.
Appendix A: T1D Cure-Oriented Stem Cell Research in Clinical Trials
Embryonic stem cells: Vertex Pharmaceuticals
In 2021, Vertex Pharmaceuticals broke ground with VX-880, the first approach to produce fully functional insulin-producing pancreatic islet cells from stem cells. This research had been in the works for over a decade under Dr. Doug Melton at Semma Therapeutics, which Vertex acquired in 2019. Subsequently, Vertex acquired its largest competitor, ViaCyte, in 2022 to accelerate its T1D stem cell cure research.
- VX-880: Phase I/II trial investigating programming ESCs into beta cells and then transplanting them into patients with T1D. Immunosuppression is required.
- VX-264: Phase I/II clinical trial utilizing the same cells in VX-880 with an added encapsulation device to protect the cells from the autoimmune system’s attack.
Adult stem cells: Creative Medical Technologies
- CELZ-201: Phase II trial investigating programmed ASCs obtained from umbilical cord tissue/blood into immune cells to protect remaining beta cells from further destruction in individuals with recently diagnosed T1D.
Induced pluripotent stem cells: Vertex (via ViaCyte)
In 2014, ViaCyte was the first company to pursue a stem cell cure with an implant containing pancreatic cells called PEC-Direct.
- VC-02: Phase II trial investigating programming embryonic stem cells into pancreatic cells, encapsulating them in a protective device, and transplanting them into patients with T1D.
