
At a Glance
- 15 for-profit entities are working to solve the T1D cell supply issue with stem cell-derived beta cells (sBCs).
- Of these, five companies have progressed their cell lines into human trials.
- Vertex Pharmaceuticals is the current lead horse with a cell therapy in phase III human trials.
- Of the five clinical trials, one is a potential Practical Cure.
February 5, 2026
Cell supply is an essential component of a future T1D Practical Cure. Today, fifteen for-profit companies are developing sBCs for T1D.
Ultimately, a Practical Cure will need to combine a sustainable supply of insulin-producing cells with a way to protect those cells from the autoimmune attack. Cell supply research efforts have largely been taken up by commercial enterprise, while cell protection is still fighting through earlier stages of development.
Unlike pure academic environments, where publishing and grant-seeking are primary objectives, companies have a strong motivation to get a product to market. For-profits have constant pressure to deliver products to patients and the marketplace as soon as possible.
The companies below were identified by analysis conducted in January 2026. The list does not include companies in earlier stages of development (bench, in vitro, etc.). This report is an overview and is meant to be representative, not fully comprehensive.
Why Is Cell Supply Important?
In established T1D, a person’s insulin-producing beta cells have been mistakenly destroyed by the autoimmune attack. Insulin is an essential hormone that the body cannot live without, which is why external insulin injections must be taken frequently to compensate. One method of restoring the body’s insulin production is to transplant new beta cells from an outside source.
Today, the only FDA-approved cell source is from cadavers. Availability is limited to only the most uncontrollable cases of T1D, and the cost is prohibitive for most. There are only a small number of donors and a handful of centers that conduct this procedure. Cadaver-sourced cells are not scalable.
On the other hand, stem cell-derived beta cells are scalable. There is clinical evidence to support that these cells can be indefinitely replicated with consistent quality and function. The concept has been proven; now we start down the road to manufacturing these cells at scale.
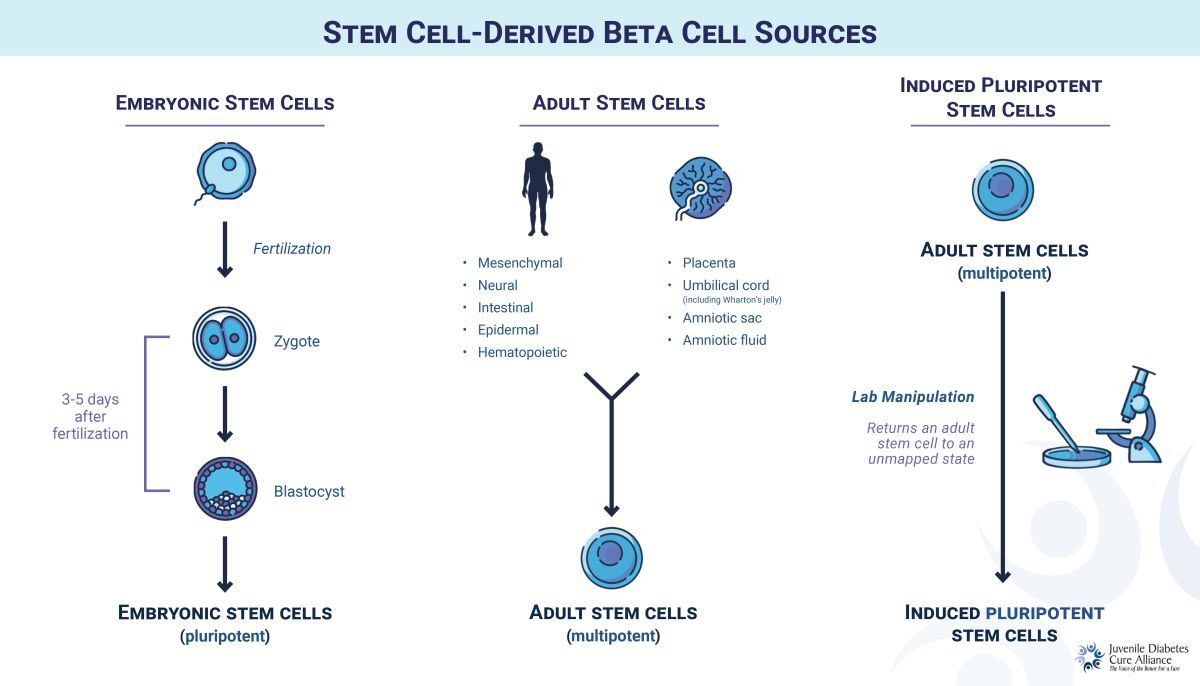
Stem Cell Types
For T1D, stem cells are engineered in the lab to become functional beta cells. There are three main sources of sBCs:
- Embryonic (ESC): Gathered from donated, pre-embryonic cells 3-5 days after fertilization (see “Stem Cell-Derived Beta Cell Sources”). ESCs can be replicated indefinitely, offering a potentially unlimited supply of beta cells, but are subject to moral challenges and controversy from some religious groups.
- Adult (ASC): Derived from adult tissue, these can develop into a limited number of cell types but cannot be replicated indefinitely. This cell type was commonly investigated a decade ago, but most researchers have turned to iPSCs as a more flexible source.
- Induced Pluripotent (iPSC): Adult stem cells are reprogrammed into an earlier, unmapped state, with the potential to become any cell type. Like ESCs, these can be replicated indefinitely, but without controversy.
The 15 Companies: Key Numbers
By the Numbers:
- 5 projects have progressed into human clinical trials.
- 11 cell lines use iPSCs.
- 3 cell lines use ESCs.
- The majority of projects source stem cells from outside of one’s own body.
- 11 projects use only allogeneic cells (from someone else).
- 2 projects use only autologous cells (from one’s own body).
- 1 project is exploring both allogeneic and autologous stem cells.
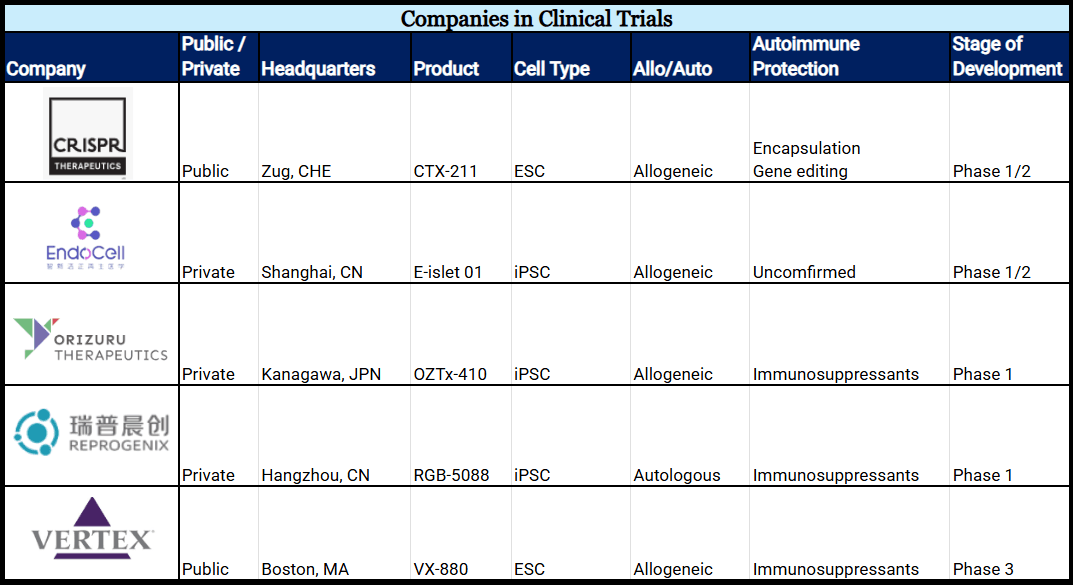
Five Companies in Human Trials
Five companies are testing sBC products in human clinical trials.
1. Vertex Pharmaceuticals’ product, VX-880, began in 2021 and is the most progressed cell line today. Two trials test VX-880 cells (ESCs) protected from the immune response via immunosuppression. At the twelve-month mark, 10/12 patients were reported to be insulin independent. Now in phase III, it is in the last stage before market approval. VX-880 will not be considered a Practical Cure until matched with a cell protection solution.
2. CRISPR Therapeutics’ product, CTX-211, utilizes an ESC line originally developed by ViaCyte. Initiated in 2023, the project began as a collaboration between Vertex (via ViaCyte) and CRISPR Therapeutics, before the latter took full ownership in 2024. The phase I/II Practical Cure trial tests gene-edited and encapsulated sBCs as protection against the immune system’s attack. At the twelve-month mark reported in January 2026, patients demonstrated detectable c-peptide levels, supporting transition to the next-generation product: CTX-213.
3. EndoCell’s product, E-islet 01, is testing blood-derived iPSCs in a phase I/II clinical trial. The trial was initiated in August 2025, recruiting individuals with established T1D and severe hypoglycemia. At this time, it is unknown what method of protection is being used.
4. Reprogenix’s product, RGB-5088, tests chemically reprogrammed iPSCs and is the only clinical-stage sBC line to use autologous cells. This is the same research that made headlines in 2024 for insulin independence in a twenty-five-year-old established T1D patient. The trial requires patients to take full-body immunosuppressants, and it will not be considered a Practical Cure until an alternative protection method is used. The phase I trial was initiated in February 2025.
5. Orizuru Therapeutics’ product, OZTx-410, is testing iPSCs evenly distributed in a sheet-like product designed to promote cell engraftment. The phase I clinical trial began in October 2024 and completed dosing of patient 1 in April 2025. The sheet-like product is not an encapsulation device; islets are protected from the immune system with immunosuppression.
Chart 1: Companies Developing sBCs in Clinical Trials (Alphabetical)
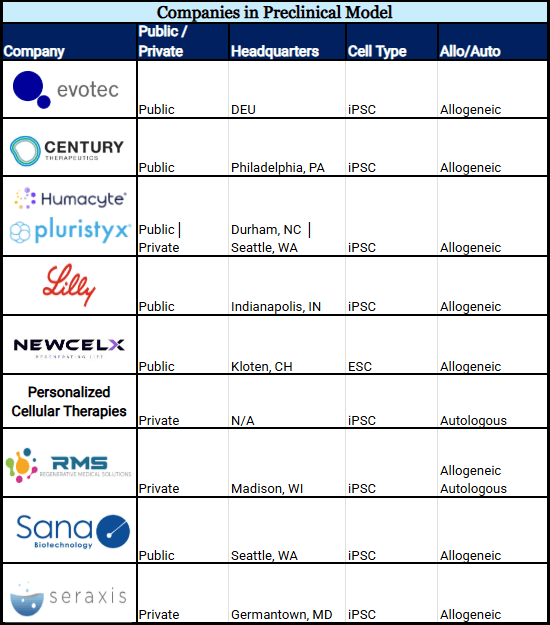
Ten Preclinical Companies
Eight stem cell lines are in preclinical animal models (see chart 2). This is the necessary stage to prove safety before transitioning to human testing.
By the Numbers:
- 8 projects use iPSCs.
- 7 projects use only allogeneic cells.
- 1 cell line is being developed as a complete collaborative effort (Humacyte & Pluristyx) where both companies contribute to sBC development.
Chart 2: Companies Developing sBCs in a Preclinical Animal Model (Alphabetical)
Collaborations
Three cell lines are being developed in collaborations. This includes companies working together to create a cell line, and in partnerships that combine individual technologies for a new project. Only collaborations where both parties participate in creating a cell line are reflected in Chart 2.
Evotec & Sernova
In 2022, the companies entered an agreement to combine Evotec’s iPSC-derived beta cell line (in development) with Sernova’s Cell Pouch. The Cell Pouch, a device currently in clinical trials using cadaver-sourced cells, is designed to house and promote the long-term health of transplanted islets.
Humacyte & Pluristyx
Collaboration began in 2024 to create gene-edited iPSCs for use in Humacyte’s investigational BioVascular Pancreas. Uniquely, both entities contribute to the development of the cell line. Pluristyx supplies the initial iPSCs and performs gene editing; Humacyte is responsible for differentiating the iPSCs into functional beta cells.
NewcelX & iTolerance
Collaboration began in 2023 to combine both entities' pre-existing technology into a new product: iTOL-102. NewcelX, formerly Kadimastem, is the sole creator of IsletRx (micro-encapsulated ESCs). iTolerance brings iTOL-100 to the table, a protective microgel that covers the transplanted islets.
