At a Glance
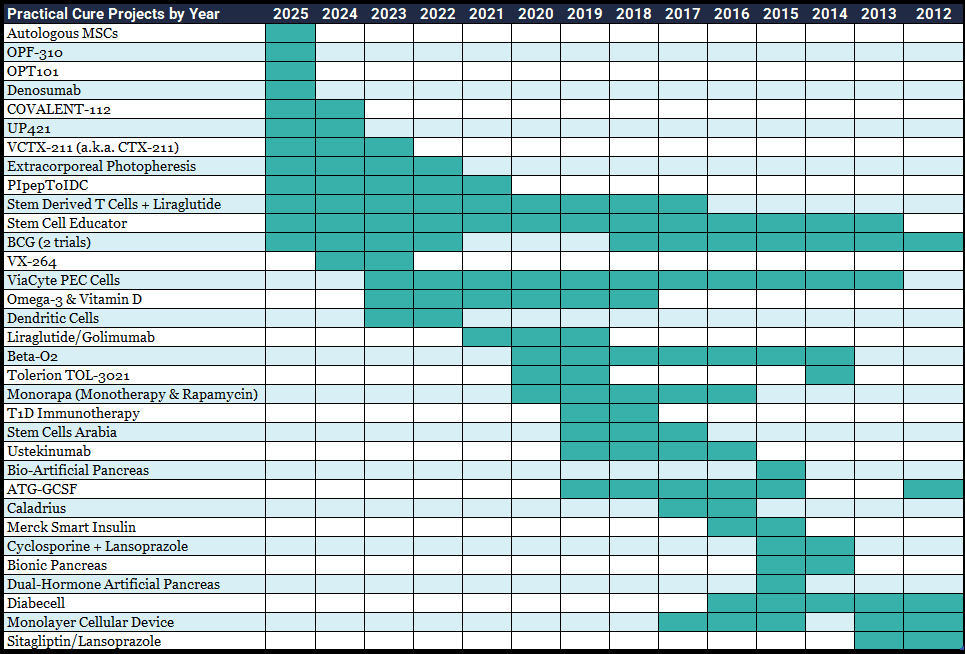
- Of 653 T1D active clinical trials, 2% qualify as potential Practical Cures.
- 12 Practical Cure projects are being tested in 13 clinical trials.
- Since our last review, four projects have been added and one removed.
September 4, 2025
As of today, there are a dozen Practical Cure projects taking place in clinical trials. These projects have progressed past the initial safety testing of animal models and are now being tested in humans. If successful, they hold the potential to become a Practical Cure for T1D within the next fifteen years.
There are several criteria that a project must meet to be a potential Practical Cure. It must be an active clinical trial registered with the US FDA, restore normal blood glucose levels with minimal monitoring, without broad immunosuppression, and enable an unrestricted diet. All projects we identified fit all of the criteria, though there are hundreds of others that only fulfill a portion.
A single project often takes place in multiple trials, assessing the same therapy in separate groups (e.g., different age, dosages, comorbidities, etc.). Information on recruiting trials is at the end of this report.
Note: This list is meant to inform and record the projects that could result in a Practical Cure for T1D. It is not an endorsement or validation of the effectiveness of the research. The results of each project, successful or unsuccessful, will speak for themselves.
New Arrivals, Continuing Projects, and a Removed Study
2025 has been an active year in Practical Cure research, with several updates since our last review in the 2024 State of the Cure for Type 1 Diabetes, published in January. At that time, there were nine Practical Cure projects. Since then, one project has left the list, five have research updates, and there are four new additions.
New Arrivals
There are four new arrivals to the Practical Cure list this year.
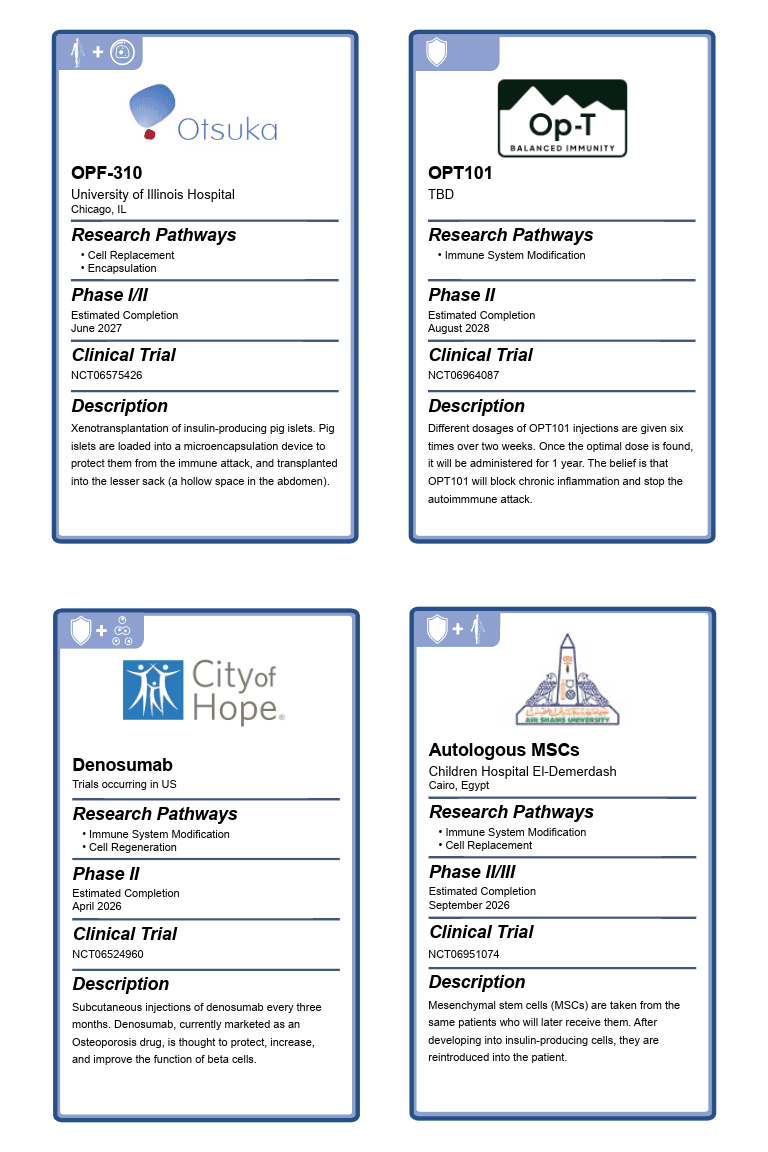
OPF-310
The phase I/II trial is testing the combination product OPF-310, the first xenotransplantation Practical Cure project since 2016. OPF-310 consists of insulin-producing pig islets, protected from the immune system with a microencapsulation device and transplanted into the lesser sack (a hollow space in the abdomen). This is a dose escalation study to determine the optimal number of transplanted islets.
The trial began June 2025 and is being tested in individuals aged thirty-five to sixty-five with unstable T1D. Owned by Otsuka Pharmaceutical Factory, the trial takes place at the University of Illinois Hospital & Health Sciences System in Chicago.
OPT101
The phase II trial tests subcutaneous injections of OPT101, a peptide-based solution that aims to regulate inflammatory pathways and stop autoimmunity, without negatively impacting the immune system as a whole.
A dose escalation study, OPT101 will be tested in three parts:
- Varying dosages of OPT101 are given three times each week for two weeks. Administered to six adult patients diagnosed with T1D within the last two decades.
- The highest tolerated dose will be tested over one year. Patients must be diagnosed within one-to-five or five-to-ten years. Some patients will receive a placebo.
- (Optional) This portion tests the highest dose in patients who are one year from diagnosis.
The study began in May 2025, and locations have not been posted. The project is owned by Op-T LLC.
Denosumab
A phase I/II trial testing repeat injections of denosumab administered once every three months for one year. Denosumab is a drug that inhibits the RANKL protein. This inhibition is believed to reduce inflammation, protect, preserve, and improve beta cell function. Denosumab is currently marketed to treat osteoporosis.
The trial began in September 2024 and is being tested in individuals diagnosed between one and five years ago. The study is led by the City of Hope Medical Center.
Autologous MSCs
A phase II/III clinical trial testing the safety and efficacy of mesenchymal stem cells (MSCs) that have been transformed into beta cells and returned to the body. MSCs are a type of adult stem cell that can be found in mature tissues. In this case, it is taken from fatty tissue in the stomach.
Autologous cells (from one’s own body) are used to avoid triggering the immune response against foreign cells. But, they are still susceptible to the attack that falsely targets beta cells.
The clinical trial description (i.e., enrollment, cell protection, time since diagnosis) is minimal. JDCA inquiries for more information were unanswered. Unless otherwise proven, this trial is included on the list as it does not mention any form of immunosuppression or other exclusionary criteria.
The trial began in April of this year and is being tested in individuals aged fifteen to eighteen. Taking place in Cairo, Egypt, it is sponsored by Ain Shams University.
Continuing Projects
Last year, nine projects took place in ten clinical trials. Of those, eight projects continue today, and five have updates.


UP421
Sana Biotechnology and Uppsala University’s UP421 project tests cadaver-sourced beta cells, gene edited so they are hidden from the autoimmune attack. The cells are then transplanted into the forearm of the recipient.
Interim results were shared last June on a single patient, a forty-two-year-old man diagnosed with T1D at the age of four. The gene-edited islets continued to survive at six months and produce insulin without immunosuppression, the first in human trial history to do so. Sana expects to file an IND to initiate another trial in 2026 testing SC451, using scalable stem cell-derived islets instead of cadaverous cells.
Umbilical T Cells + Liraglutide
Though currently marked “Unknown Status” on the US clinical trial database, inquiries confirmed that trial follow-up has been extended to July 28, 2026. Additionally, principal investigator Zhiguang Zhou added that recruitment for the study is complete, and “Based on the interim data collected, the treatment regimen has shown promising results in terms of both safety and efficacy.” Though a hopeful statement, verifiable results have not yet been published.
Repeat BCG Vaccinations for the Treatment of T1D
BCG released general, cumulative results for its ongoing clinical trials during the ADA’s 85th Scientific Sessions in June. 364 total patients with T1D have been treated in clinical trials. Multiple doses of BCG have permitted patients to see some reduction in HbA1c (10-15% in early-onset adults) for eight years, but no insulin independence is reported.
PIpepTolDC
City of Hope’s Senior Manager of Media Relations, Letisia Marquez, clarified that the study completion date would be extended to early 2026 to finalize patient follow-ups. Three patients were recruited for this study, none of whom experienced adverse safety concerns.
OPERA
Communication with Dr. Yandy Marx Castillo-Aleman, MD, the Apheresis Medical Director at ADSCC confirmed that the trial was active, ongoing, and will be extended by eighteen months. The estimated completion date is now December 2026. Though no interim results were offered, we will provide updates as they are made available.
Removed Trial: VX-264
On March 28, 2025, Vertex Pharmaceuticals announced the end of its phase I/II trial, VX-264. The project began in May 2023, testing a line of stem cell-derived beta cells protected from the immune system with an encapsulation device.
These same cells are used in Vertex’s pivotal trial, VX-880, which has resulted in insulin independence (with full immunosuppression) and is poised for FDA approval. In the case of VX-264, the protective device was not viable.
Though it proved generally safe and well tolerated, increases in C-Peptide “were not observed at levels necessary to deliver benefit.” Vertex claimed it would explore encapsulation further as well as alternative cell protection techniques.
Please note this trial has not been updated on the US trial database to reflect the changed status.
Appendix A: Practical Cure Trials Actively Recruiting
Canada: VCTX-211 (NCT05565248)
Egypt: Autologous MSCs (NCT06951074)
Sweden: UP421 (NCT06239636)
United Arab Emirates: OPERA (NCT05413005)
United States:
- BCG (NCT05180591)
- Denosumab (NCT06524960)
- OPF-310 (NCT06575426)
- Stem Cell Educator (NCT04011020)
Appendix B: Practical Cure Projects, 2012-2025