
At a Glance
- A sustainable supply of insulin-producing cells is necessary for a Practical Cure.
- Five primary research pathways addressing beta cell supply have progressed through animal and, in most cases, human testing.
- Of the five pathways, stem cell-derived beta cells (sBCs) currently seem the most promising.
- Future reports will profile leading sBC companies, research initiatives, and address cell protection research pathways.
May 2, 2025
A Practical Cure (a.k.a. Functional Cure) for T1D requires two fundamental components working hand-in-hand: 1) a sustainable supply of insulin-producing cells, and 2) a method to protect them from the autoimmune attack. This report summarizes the major research pathways addressing cell supply as part of a T1D Practical Cure. A later report will address cell protection pathways.
Cell supply pathways are in various stages of development, with commercial enterprises taking center stage with stem cell-derived beta cells (sBCs). The lead horse, Vertex Pharmaceuticals, recently started stage three human trials, the last step before FDA approval. Other competitors are quickly joining the field.
There are five main cell sources utilized in current research:
- Cadaverous (recently deceased donors)
- Stem Cell-Derived Beta Cells (human stem cells)
- Residual (replenishing the small number of beta cells still in the body)
- Xenogeneic (other animals, namely pigs)
- Transdifferentiation (reprogramming non-insulin-producing cells into beta cells)
The most advanced research pathway is sBCs, attracting the most attention and competition. There are alternative theories and methods beyond those here to address cell supply, but none have progressed beyond the bench and therefore are not included in this report.
Cell protection research pathways and the most developed sBC companies will be addressed in similar overviews in the weeks ahead.
1. Cadaverous
Cadaverous cells—pancreatic islets harvested from organ donors—have been the only available source of beta cells for years. Most islet transplantation research and procedures conducted to date have used cadaver cells.
However, this cell type is extremely limited in availability and is prohibitively expensive. It is entirely dependent on the availability of donor islets. Donor quality and cell potency are not guaranteed, and patients require multiple transplants, needing more cells.
In summary, cadaverous islet transplants are restricted to individuals with the worst cases of the disease and are not a viable source for the broader T1D population.
2. Stem Cell-Derived Beta Cells (sBCs)
The stem cell-derived beta cells research pathway is the most promising and prolific source of cell supply. There are a few differences that are important to understand.
My Stem Cells or Someone Else’s?
Stem cells originating from the same person who later receives them are autologous. Stem cells originating from another person are allogeneic.
The benefit of autologous cells is that they are seen as part of the body and avoid the immune attack against foreign cells. However, these calls are only available on a person-to-person basis—one person and one procedure at a time. Autologous cell procedures are complicated, expensive, and cannot be scaled into a widely available product. We expect these procedures, if successful, will be limited to those with uncontrolled T1D.
On the other hand, Allogeneic cells can be mass produced at scale. These stem cells are developed from a single donor and can be replicated indefinitely. Allogeneic beta cells might one day become a packageable product that is widely available.
It is worth noting that both types are susceptible to the T1D autoimmune attack, and a cell protection solution will be needed.
Where Do Stem Cells Come From?
In T1D, stem cells are engineered in the lab to become functional beta cells. There are three main types of sBCs, each with advantages and disadvantages.
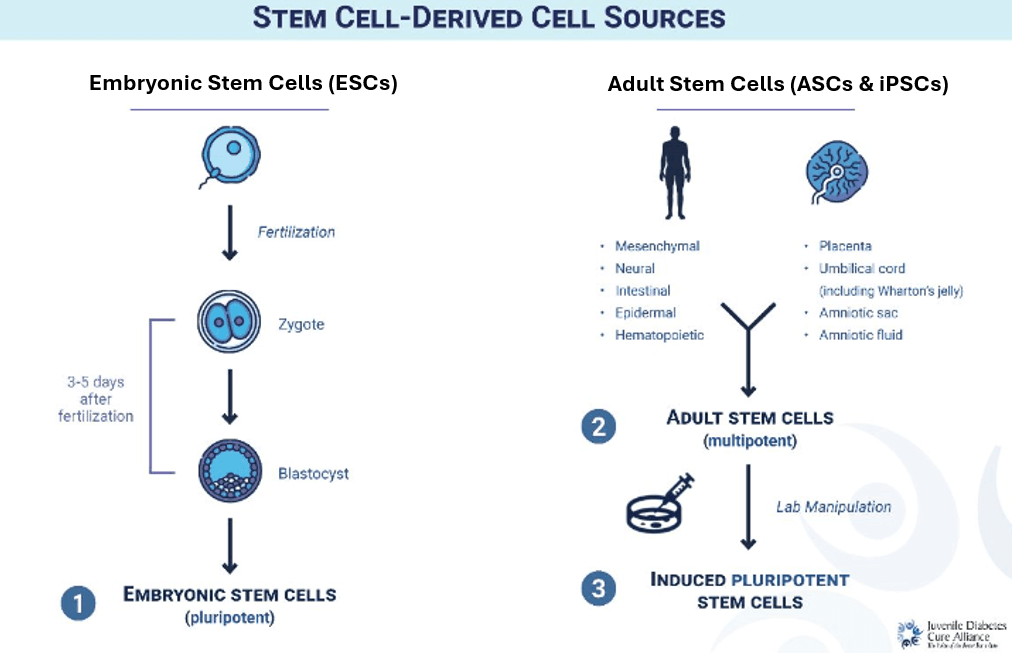
Embryonic (ESC): Stem cells are gathered from donated, pre-embryonic cells 3-5 days after fertilization, once cells reach the blastocyst stage (see “Stem Cell-Derived Cell Sources”). These can be trained to develop into any cell, replicated indefinitely, and offer a potentially unlimited supply of beta cells. Research demonstrates that these cells transplanted into adults with fully established T1D can result in insulin independence, but not yet at scale.
Adult (ASC): Stem cells are derived from adult tissue in different parts of the body. These are multipotent, meaning they develop into a limited number of cell types. These cannot be replicated indefinitely.
Induced Pluripotent (iPSC): Adult stem cells are reprogrammed into an earlier, unmapped state. Once they are ‘blank slates’ without a specific job, they can develop into any cell type and be replicated indefinitely.
3. Residual
Though somewhat debated, most data suggest a small amount of residual beta cells remain in the body decades after diagnosis, hidden from the autoimmune attack. In theory, these cells should be able to replicate and regenerate. This research has been underway for several decades.
Numerous research projects are attempting to modulate the immune attack while boosting the regeneration of these cells. One of the well-known and long-running projects working on this pathway is BCG.
To date, no project focused on regenerating residual beta cells has resulted in insulin independence or appears close to a pivotal phase III trial.
4. Xenogeneic
‘Xenogeneic’ refers to sourcing beta cells from non-human species, primarily pigs. This source offers a readily available, scalable, and inexpensive supply of cells. As a result, this was one of the first cell supply pathways undertaken nearly forty years ago and was a major focus of research activity during the 1980s and 1990s.
However, because these cells do not originate from the same species, let alone person, the immune system identifies them as foreign invaders and initiates an especially strong attack. While there has been some success for this pathway in mice, it has not been successful in humans.
A few companies continue to pursue this pathway and claim they are bringing a different approach than in the past. One well-known example is eGenesis, an offshoot of a Harvard academic laboratory, but it is unclear if they are still focused on T1D.
Overall, xenogeneic islets have not yet resulted in long-term insulin independence, and there are no US projects in human trials.
5. Transdifferentiation
In a laboratory setting, non-insulin-producing cells are transformed into beta cells. This happens through gene editing, protein manipulation, and/or drugs. The most common cell type targeted is the alpha cell, responsible for glucagon (glucose) production, as beta cells originate from the same pancreatic progenitor cells.
Transdifferentiation is currently in animal models, with no clear path to human clinical trials. This cell type is still susceptible to autoimmune attack, and the new beta-like cells’ functionality and long-term survival are significant hurdles.
