March 15, 2022
This January, we published the 10th annual State of the Cure for Type 1 Diabetes. In recognition of this decennial anniversary, we take a moment to reflect on the progress of the past ten years toward achieving a Practical Cure for type 1 diabetes. During this period, T1D donors gave roughly $5 billion to T1D non-profit organizations and research centers. This report will address the return on that investment.
THE MAIN FINDING: No Practical Cure but Some Promising (but slow) Developments
The big finding from this report is that managing T1D is a major burden today, as it was ten years ago. Despite some promising developments, the T1D research ecosystem lacks the sense of urgency towards a cure that has defined many of the great scientific leaps forward of the 20th and 21st centuries.
This report will cover four key topics: 1) Trends in T1D Donor Priorities; 2) Practical Cure Research in Human Trials, 3) T1D Nonprofit Resource Utilization, and 4) Notable Advances.
T1D DONOR PRIORITIES: Cure is Still Job #1
One thing that has not changed over the decade is that the number one priority of T1D donors is to fund cure research. In 2011, 90% of survey respondents said that funding a T1D Practical Cure was the number one reason they donated (1); in 2021, 86% said funding a cure was the number one reason (2). No other reason comes close.
JCDA Survey of T1D Donor Priorities, 2021
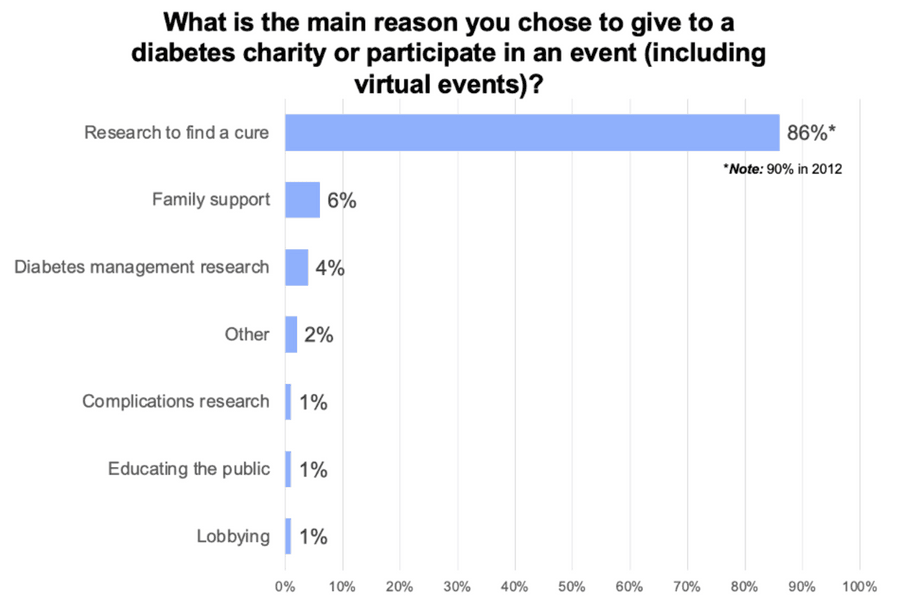
Accordingly, it is no surprise that the number one marketing promise used in fundraising solicitations is that all money raised will fund cure research. This trend has remained unchanged over the past decade. In 2012, 95% of JDRF’s fundraising events solicited donations with a cure promise (3); in 2021, 93% of JDRF’s fundraising events used a cure promise (4). In 2012, 89% of the American Diabetes Association (ADA) fundraising events solicited donations with a cure promise (3); in 2021 96% of the ADA’s fundraising events used a cure promise (4).
PRACTICAL CURE RESEARCH IN HUMAN TRIALS: Still not enough
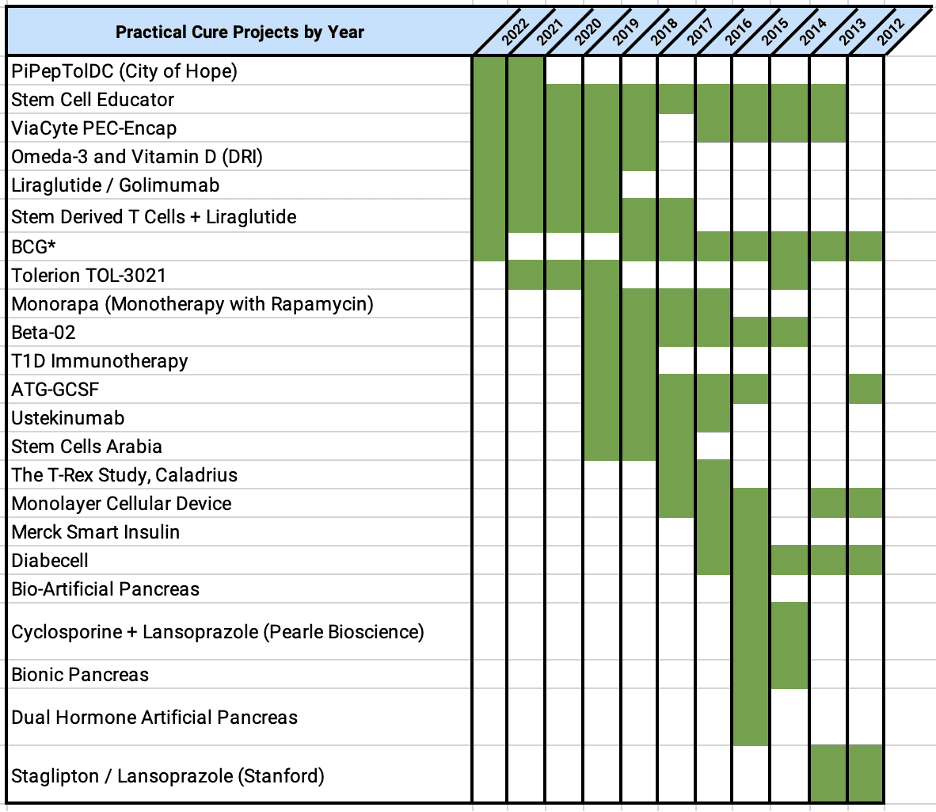
In the simplest terms, a T1D Practical Cure trial is any human trial whose aim is to substantially reduce or eliminate the burden of managing T1D for people living with the disease today. In 2012, out of 329 T1D human trials registered in the FDA database, just 5 were testing a potential Practical Cure (5). In 2021, there were twice as many T1D human trials registered with the FDA, but only 12 Practical Cure trials – still less than 2% of the total (6). A paradigm shift is still needed today, just as it was in 2012.
The 12 trials today are testing seven different projects. The following chart shows the evolution of Practical Cure projects in human trials by year:
T1D NONPROFIT RESOURCE UTILIZATION: A big, sustained drop in research grants
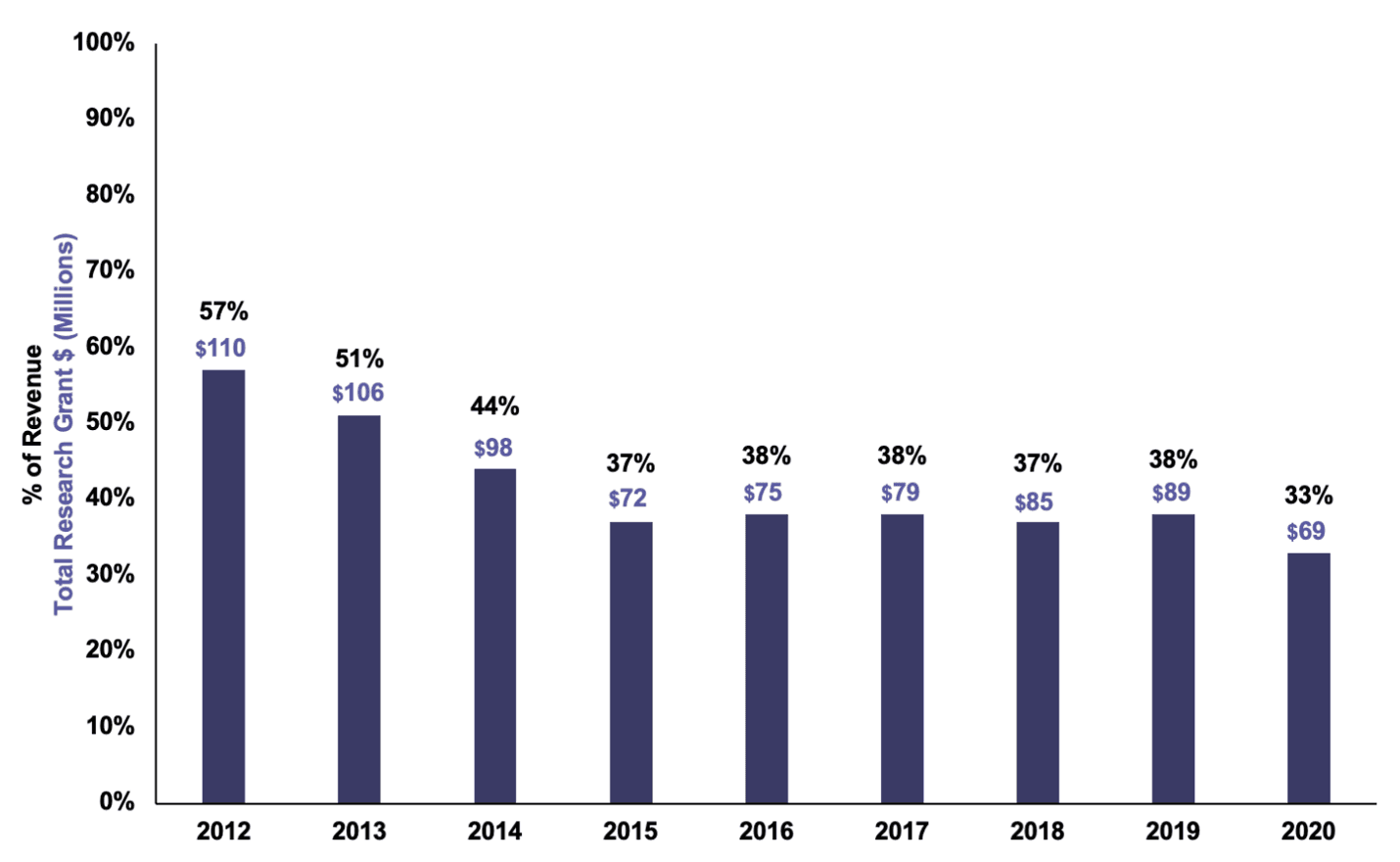
Over the past ten years, T1D nonprofits have shifted their focus away from funding research grants for T1D. In 2012, JDRF wrote $110 million of T1D research grants, 57% of its revenue. That number has steadily declined: in 2021, JDRF dedicated $69 million to T1D research grants, just 33% of its revenue (see chart below).
JDRF Research Grant Spending as % of Revenue, 2012-2021
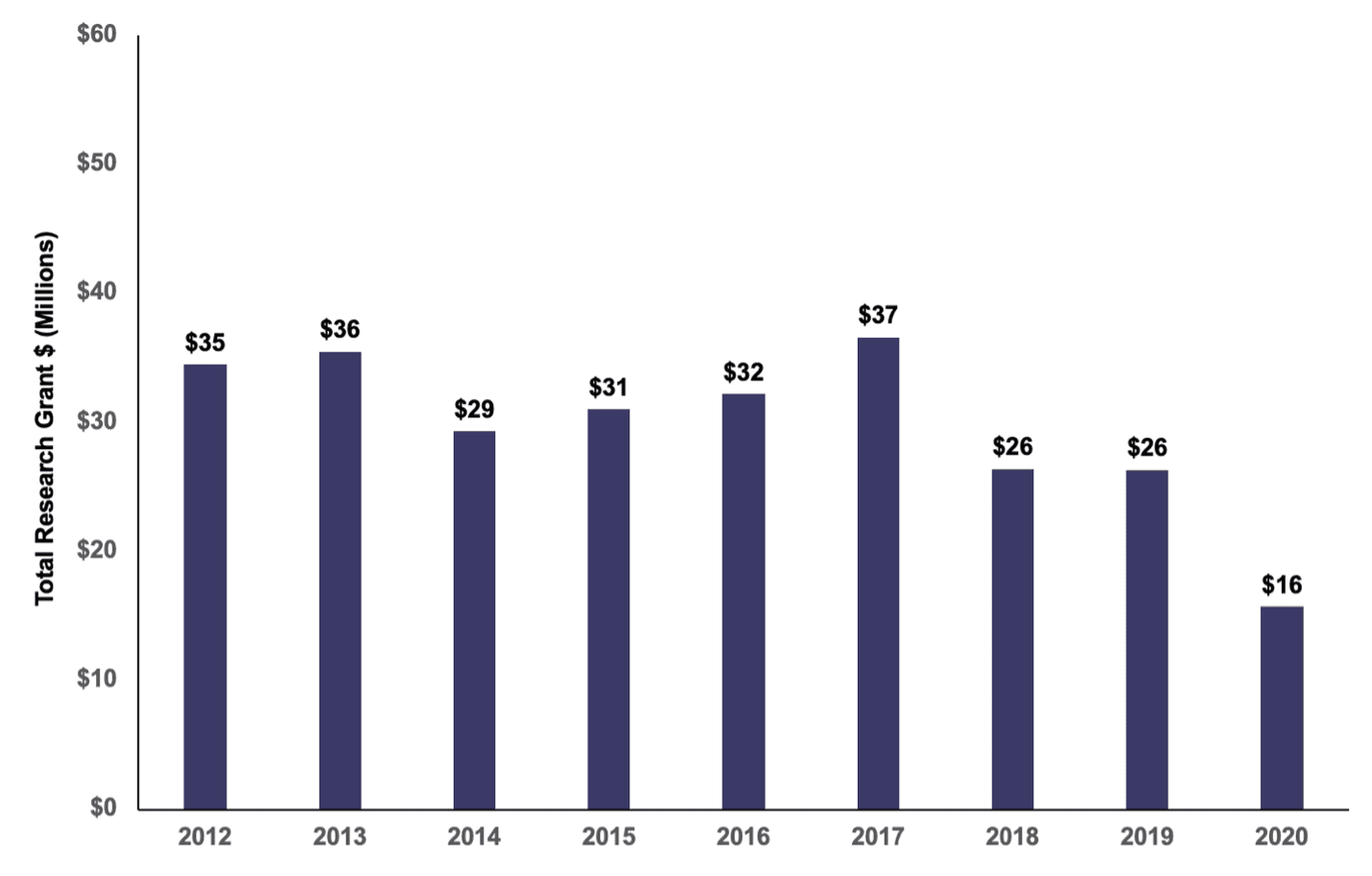
The ADA, meanwhile, has steadily dedicated around 15% of its revenue to fund diabetes research grants. However, as the ADA’s revenue has steadily declined over the decade, so too has the amount it dedicates to research (see chart).
ADA Research Grant Expense (in Millions), 2012-2021
This shift away from research grant spending stands in stark contrast to the promise used to raise money, as discussed in section #1. Even though only a minority of money is used to fund research grants, the primary fundraising promise is that the money will be used for cure research. The lack of alignment with donor priorities has been present for the past decade and has only deepened in recent years.
NOTABLE ADVANCES: Opportunities for Hope (and a renewed focus on cure research)
Despite the structural roadblocks, there have been some advances in medical research pathways that may lead to a Practical Cure. Additionally, there has been some true innovation in how we fund research.
Stem Cells as Cell Supply
Ten years ago, the hope was that islet cells extracted from pigs could replace the need for people with T1D to manually administer insulin. While “porcine cells” have not fulfilled their potential, researchers showed last year that islet cells derived from embryonic stem cells could become functioning beta cells and reduce or replace the need for people to take external insulin. If this research holds up to the rigor of a large clinical trial testing efficacy, then the research focus will finally be able to shift to protecting these cells without the need for immunosuppressive drugs.
Currently, two companies have active clinical trials of their stem-derived islets. The Boston-based Vertex and the San Diego start-up ViaCtye are each racing towards a Practical Cure.
And, just a few steps behind, a group of aspiring companies and academics are testing stem cell-derived islet cells in animals. They too will be seeking to enter human trials in the years ahead. These developments are exciting.
Gene Editing and Other Advanced Cell Protection Techniques
Ten years ago, the idea that we could identify, pinpoint, contact, and edit (“fix”) a bad-actor gene was the stuff of science fiction. Today, the latest evolutions in gene therapy and CRISPR-Cas9 may pave the way to shield insulin-producing cells from immune rejection. We are still early days of this research, but it is an exciting and promising research frontier.
A New Research Funding Model that Rewards Focus and Urgency
The launch of the JDRF T1D Fund in 2016 brought a unique, innovative, and high-impact approach to funding T1D research projects. Unlike JDRF, which mainly gives grants to academic researchers, the T1D Fund uses donor capital to invest in early-stage companies with T1D commercial potential. They do not give grants; they invest in exchange for ownership equity.
Should the company reach the market, or be acquired, the Fund receives a payout which it can re-invest. As a result, the projects it invests in are on a fast-paced timetable to achieve commercial viability as soon as possible. Several of the Fund’s early investments address aspects of a Practical Cure (7).
Advances in the Mechanical Artificial Pancreas
Ten years ago, continuous glucose monitors were not reliable enough to link with insulin delivery systems to make day-to-day T1D treatment decisions. However, the latest iterations of CGMs are smaller and more predictive than they were in 2012. Furthermore, many of us live with “smart” connected devices today, and it is not difficult to imagine a future where the countless treatment decisions that a person with T1D faces are mitigated by a smart mechanical solution.
Nobody would argue that the latest “partial closed-loop” artificial pancreas systems sold in the US by companies like Medtronic, Insulet, and Tandem qualify as a Practical Cure for T1D. These devices are not small enough or smart enough to allow the user to “set it and forget it.” However, the advances we have seen in continuous glucose monitors and advanced insulin delivery systems over the last ten years suggest a future product that could potentially eliminate the burden of the disease.
