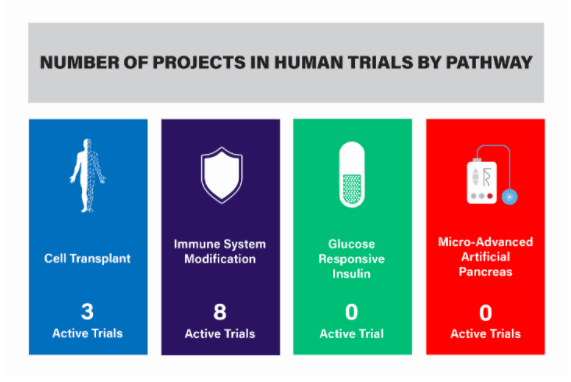
This report reviews the 11 active Practical Cure (PC) research projects underway in United States FDA approved human clinical trials. A summary of these projects is presented in the charts below by pathway and includes the project title, description, location, and status. A full definition of a Practical Cure, as well as the four research pathways, can be found in last week's JDCA report, The Four Research Pathways to a Practical Cure for Type 1 Diabetes (Click here to view).
Three of the eleven Practical Cure research projects are new to the list while three projects from 2017 have been abandoned.
Beyond the 11 active projects, there is also an appendix at the end of the report which outlines high-profile emerging Practical Cure projects. These projects are 'emerging' because they are not currently testing in human trials but are moving quickly in that direction. Our coverage of emerging projects is limited to well known, high profile projects and is not meant to be fully comprehensive.
Projects by pathways are listed below.
IMMUNE SYSTEM MODIFICATION WITH SUSTAINABLE CELL SUPPLY
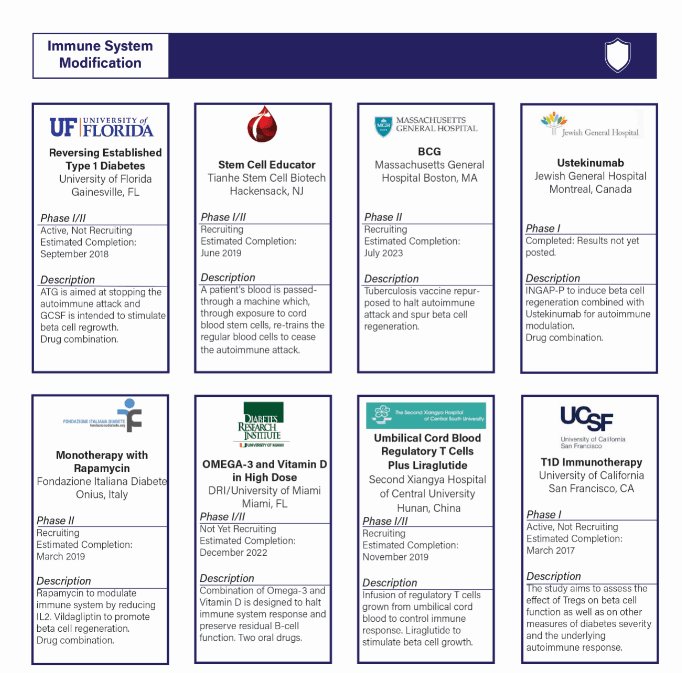

Definition:
- Therapy to stop the immune system from destroying beta cells, including modifying, blocking, and retraining.
Commentary:
- This pathway has the largest number of active trials and has seen an increase in active trials over the last five years. One concern within this pathway is immune system modification research is often targeted at T1D prevention or early onset intervention, as opposed to finding a cure for those currently living with the disease.
- There are eight active trials, all of which are ongoing.
- One project, Caladrius has been abandoned since last year because it is testing in early-onset. If this research moves into testing for established T1D it will be considered a Practical Cure.
CELL TRANSPLANT WITH AUTOIMMUNE PROTECTION
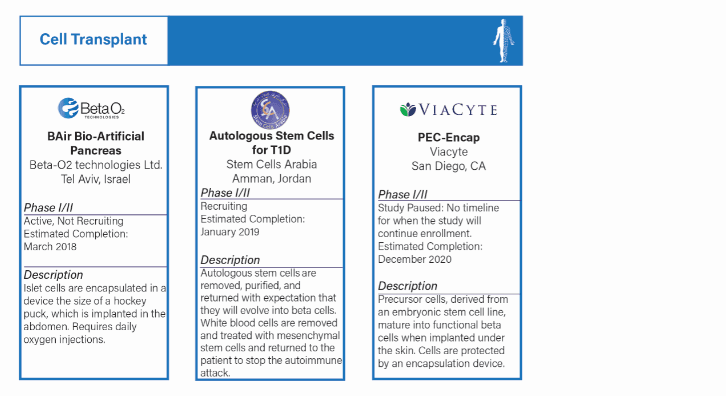

Definition:
- Implanting islet cells, stem cells, or precursor cells to achieve insulin independence. Cells are protected by an encapsulation device or immune system modification.
Commentary:
- Over the past decade, there have been significant advances in islet, stem, and precursor cell development and production. The remaining hurdle involves the development of an encapsulation device or sustainable long-term immune system modification to protect the cells from the body's immune response.
- There are three active trials, all of which are ongoing.
- One project, Monolayer Cellular Device at Cliniques Universitaires Saint-Luc-UCL has been abandoned since last year because it has been concluded without results for more than three years.
GLUCOSE RESPONSIVE INSULIN and MICRO-ADVANCED ARTIFICIAL PANCREAS

Definitions:
Glucose-Responsive Insulin: Insulin which chemically activates in response to changes in blood sugar.
Micro-Advanced Artificial Pancreas: A device that mimics the pancreas by monitoring changes in blood sugar and independently administers insulin without the patient’s input. To be considered a Practical Cure the device must be small enough for users to completely forget about its presence. No current devices are small enough to meet this requirement.
Commentary:
Smart Insulin has seen advances over the past few years and a number of approaches are being pursued, including smart insulin patches, intranasal insulin, as well as the traditional pill form.
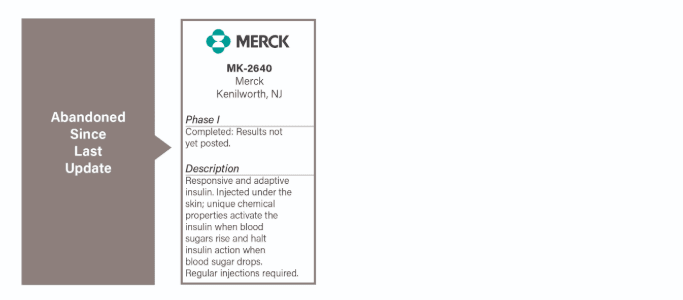
Merck's smart insulin human trial, the only smart insulin trial which had been approved for testing, has been abandoned because it has been concluded without results for more than three years.
No artificial pancreas projects in, or nearing, human trials are small enough to be considered a Practical Cure. However, technology can evolve quickly and we remain optimistic about future progress.
APPENDIX A: EMERGING PRACTICAL CURE PROJECTS
Emerging PC projects include research which may begin human trials in the next two years. The projects listed below have received a significant amount of media coverage and represent the next-in-line PC projects. However, because none of these projects are currently testing in humans, they are likely more than 15 years away from FDA approval.
This list is not comprehensive and the JDCA welcomes further submissions. If you would like to submit another trial for consideration please email info@thejdca.org.
CELL TRANSPLANT WITH AUTOIMMUNE PROTECTION
- PharmaCyte Biotech has begun testing its Cell-in-a-Box microencapsulation technology with insulin-producing melligen cells, derived from liver cells. It is still in preclinical testing.
- Orgenesis has developed a process to convert a patient’s liver cells into insulin-producing cells. Two clinical trials are scheduled to start in Germany and Belgium in 2018.
- Sernova is developing an encapsulation “pouch” the size of a credit card. The company is currently testing its device with immunosuppression.
- Semma Therapeutics is commercializing Doug Melton’s work using stem cells to create beta cells. An encapsulation therapy is still in preclinical testing and development.
- Encellin, a biotechnology startup, has licensed an encapsulation device the size of a quarter from UCSF.
IMMUNE SYSTEM MODIFICATION
- DiaVacs is working on a reverse vaccine to stop the autoimmune attack. The company plans to initiate clinical studies in 2018.
- Imcyse is developing an immune system modification therapy which employs modified peptides to alter T cell behavior. The company was scheduled to start an 18-site trial in 2017 but terminated the trial due to manufacturing issues. There is currently no timeline to start clinical trials.
- Sitagliptin and Lansoprazole is a drug combination designed to regulate the autoimmune response and stimulate beta cell growth. An early-onset trial was completed in 2017.
- Neovacs is developing a vaccine designed to stimulate T regs and stimulate beta cell growth. It is currently testing in mice.
- City of Hope is developing a vaccine designed to reset the autoimmune attack and stimulate beta cell growth. It is currently in preclinical testing at a collaborating university in the Netherlands.
- Tolerion TOL-3021 is developing a reverse vaccine, similar to BCG, which increases T reg cells and stimulates beta cell growth. A phase 2 trial was completed in 2012. There is no current timeline for a phase 3 trial.
GLUCOSE RESPONSIVE INSULIN
- Sanofi is working on smart insulin solutions in collaboration with MIT, UNC, and others. There is no current timeline for moving into clinical trials.
- Merck is developing a responsive and adaptive insulin. The initial product, MK-2640 failed, but Merck is currently working on another product candidate.
- Case Western Reserve University is developing a rapid-acting, glucose-responsive insulin that activates when blood sugar is high and becomes inactive when blood sugar is low. There is no current timeline for moving into clinical trials.
ARTIFICIAL PANCREAS PROJECTS
- Currently no emerging PC projects.
