SUMMARY:
- The Caladrius Biosciences Inc. T-regulatory ("Treg") cell-based therapy is one of twelve T1D Practical Cure projects currently in human trials.
- This approach hopes to address the autoimmune response by increasing regulatory T cells manufactured from a person's own blood supply.
- The initiative is progressing through human trials:
- A phase 1 safety trial of children with early-onset T1D (diagnosed within a year) was completed in 2014.
- A phase 1 safety trial of adults with established T1D was completed in 2015.
- A Phase 2 efficacy trial of adolescents with early-onset is currently recruiting trial participants.
- Project leaders may be shifting the research focus away from a solution for established T1D to a solution for early-onset.

This is the third in a series of reports detailing individual research projects currently in human trials with the potential to deliver a Practical Cure. The twelve Practical Cure projects that will be profiled were identified and summarized in the 2016 State of the Cure for Type 1 Diabetes, which was published during the first week of January 2017.
This report focuses on the Caladrius Bioscience Treg project (CLBS03) and features an interview with Caladrius CEO and Director, David Mazzo. Caladrius is a for-profit company located in New Jersey active in stem cell therapy, regenerative medicine, and cardiovascular disease.
RESEARCH OVERVIEW
Caladrius' Treg project is attempting to eliminate or, at a minimum, reduce insulin dependence by balancing different types of T cells. The working theory is that people with T1D have an insufficient amount of regulatory T cells (Treg) compared to effector T cells, resulting in an imbalance in the immune system. If this balance is restored by increasing the number of regulatory T cells than the body will, according to the theory, stop attacking beta cells.
Research participants undergo a three-part procedure. First, blood is drawn from the patient and the Treg cells are isolated and separated. Second, those Treg cells are used to manufacture a significant volume of additional Treg cells. Last, the manufactured Treg cells are mixed back into the drawn blood and returned through an IV to the person's body (See graphic below). One key benefit of this process is that it uses the person's own blood supply which minimizes the introduction of any foreign substance into the body.
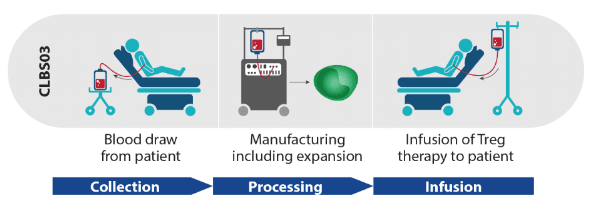
Source: Caladrius
“We wanted to bolster the immune system in a way that would allow it to stop the disease progression," Mazzo says. "If you can catch people when they still have some functioning islets and balance their immune system, then you can potentially postpone or eliminate disease progression, and give those dormant (Beta) cells an opportunity to become non-dormant. Then you give people the opportunity to live a quasi-normal life, very much external insulin sparing, or maybe even eliminate the need for external insulin.”
CURRENT TESTING:
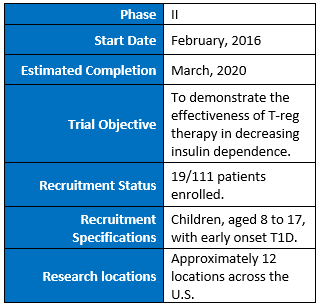
The current phase II trial is being conducted in conjunction with Sanford Health, a healthcare and research system headquartered in South Dakota. The phase II trial is commonly referenced to as The Sanford Project: T-Rex study in the press. This title refers only to the phase II trial, and not the overall Caladrius initiative.
Although phase I testing focused on adults with established T1D, the current phase II research has shifted focus to adolescents who were diagnosed within the past 12 months. The trial is currently recruiting 111 patients, which Caladrius expects to complete by the end of 2017.
The objective of the trial is to reduce or stabilize the amount of external insulin that participants require. If successful, C-peptide levels are expected to stabilize or increase. Glucose, A1C levels, and hypoglycemic episodes will also be tracked. Patients will participate in four visits in the first month following the infusion procedure and six visits over the following two years.
HUMAN TRIALS RESEARCH DESIGN SUMMARY (ACTIVE):
Clinical Trials Link: https://clinicaltrials.gov/ct2/show/NCT02691247
Caladrius Website Link: http://www.caladrius.com/product-candidates/clbs03/
Sanford Research Website Link: http://www.sanfordhealth.org/promo/the-sanford-project
