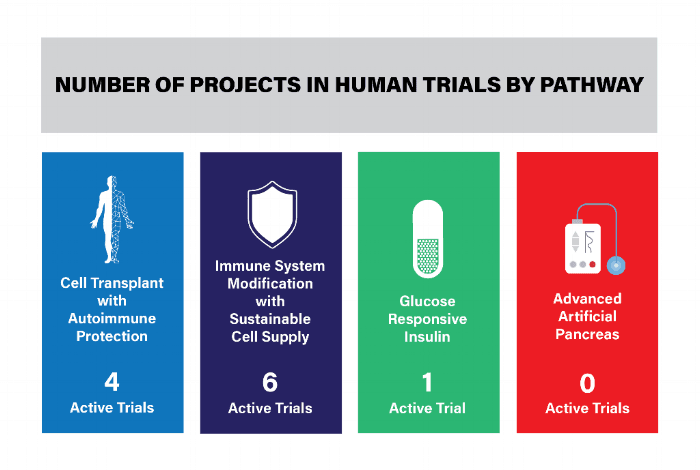
This report reviews the 11 active Practical Cure (PC) research projects underway in FDA approved human clinical trials. A summary of these projects is presented in the charts below by pathway and includes the project title, description, location, and status. A full definition of a Practical Cure, as well as the four research pathways, can be found in last weeks’ report, The Four Research Pathways to a Practical Cure for Type 1 Diabetes (Click here to view).
Each of the four PC research pathways represents a different approach to the same endpoint; minimizing the disruptive aspects of T1D. Trials within the same pathway are pursuing comparable research strategies.
The main takeaway is that no new Practical Cure projects have been added in 2017 and one project from 2016 has been abandoned.
Beyond the 11 active projects, there is also an appendix at the end of the report which outlines high-profile emerging Practical Cure projects. These projects are 'emerging' because they are not currently testing in human trials but are moving quickly in that direction. Our coverage of emerging projects is limited to well known, high profile projects such as research being done at the DRI Biohub and Semma Therapeutics.
Projects by pathways are listed below.
CELL TRANSPLANT WITH AUTOIMMUNE PROTECTION
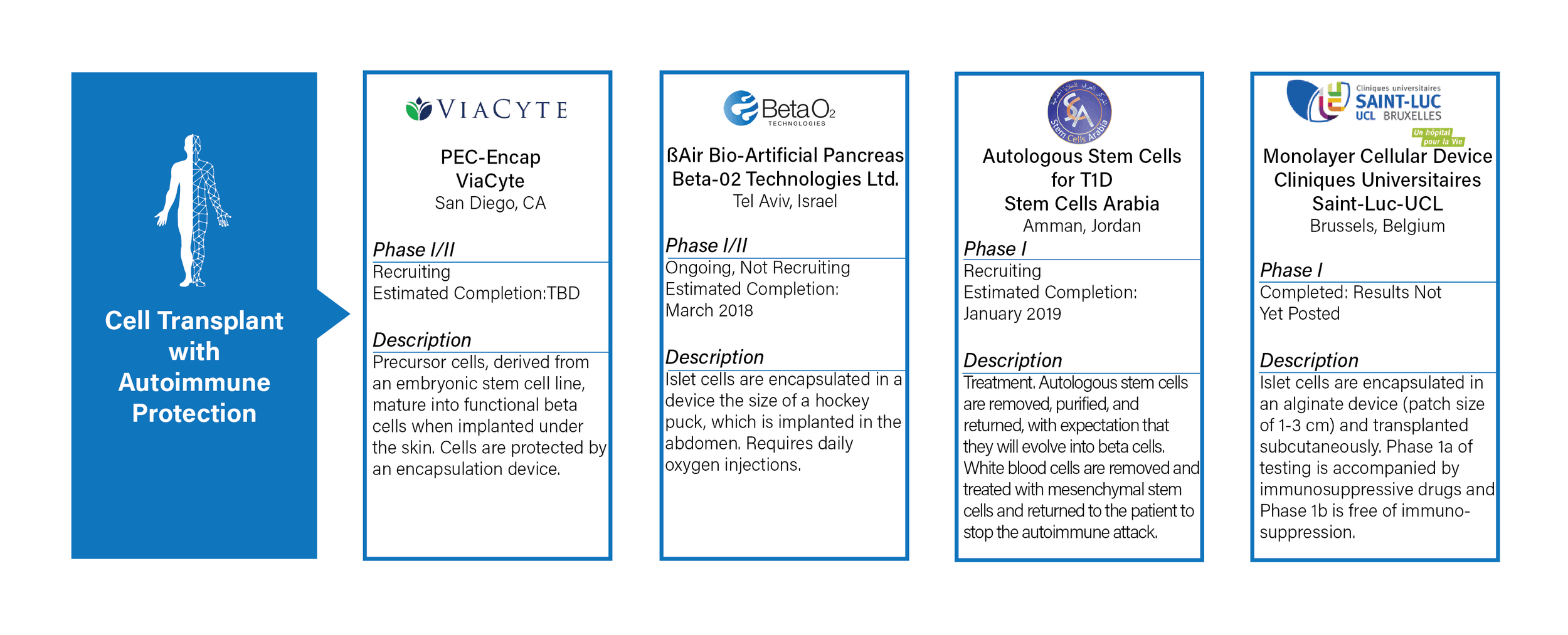
- Definition: implanting islet cells, stem cells, or precursor cells to achieve insulin independence. Cells are protected by an encapsulation device or immune system modification.
- Commentary: Over the past decade, there have been significant advances in islet, stem, and precursor cell development and production. The remaining hurdle involves the development of an encapsulation device or immune system modification requirement that is sustainable long term.
- There are four active trials, three are ongoing and one is completed but has not yet posted results.
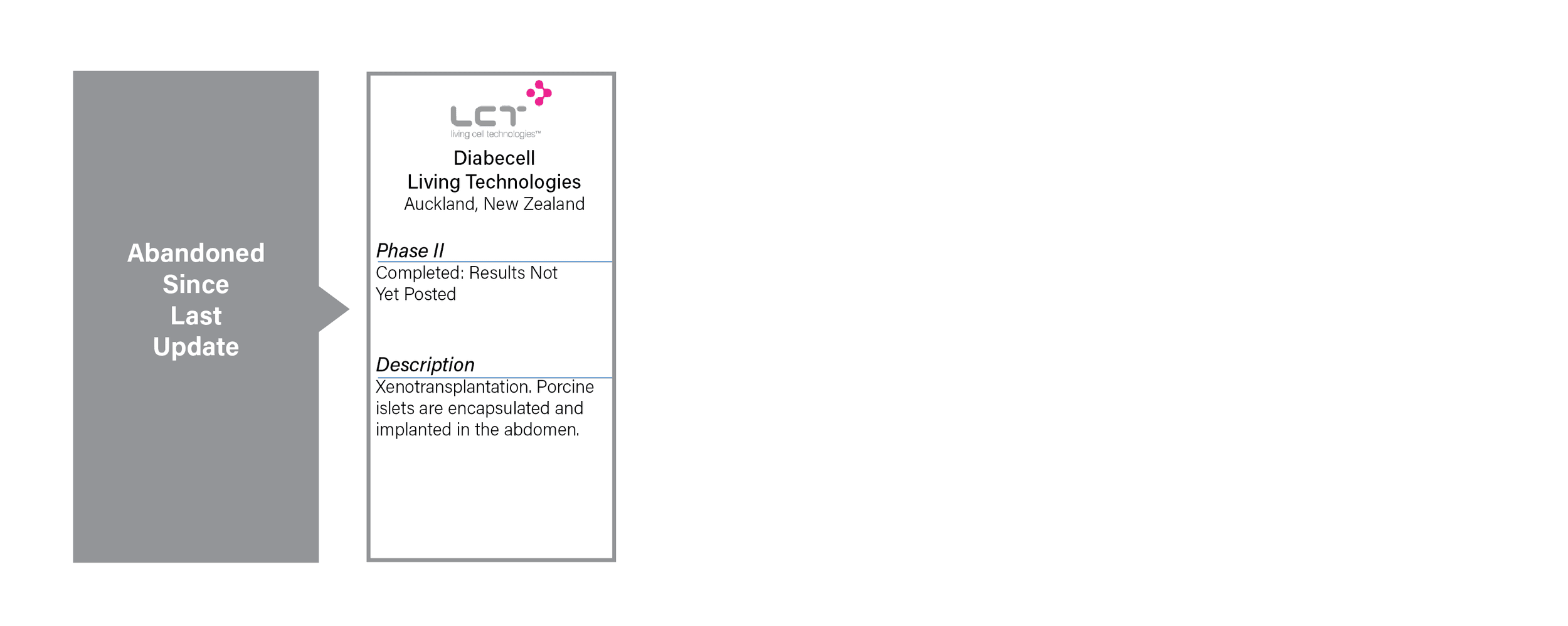
- One project, Diabecell has been abandoned since last year because it has been concluded without results for more than three years.
IMMUNE SYSTEM MODIFICATION WITH SUSTAINABLE CELL SUPPLY
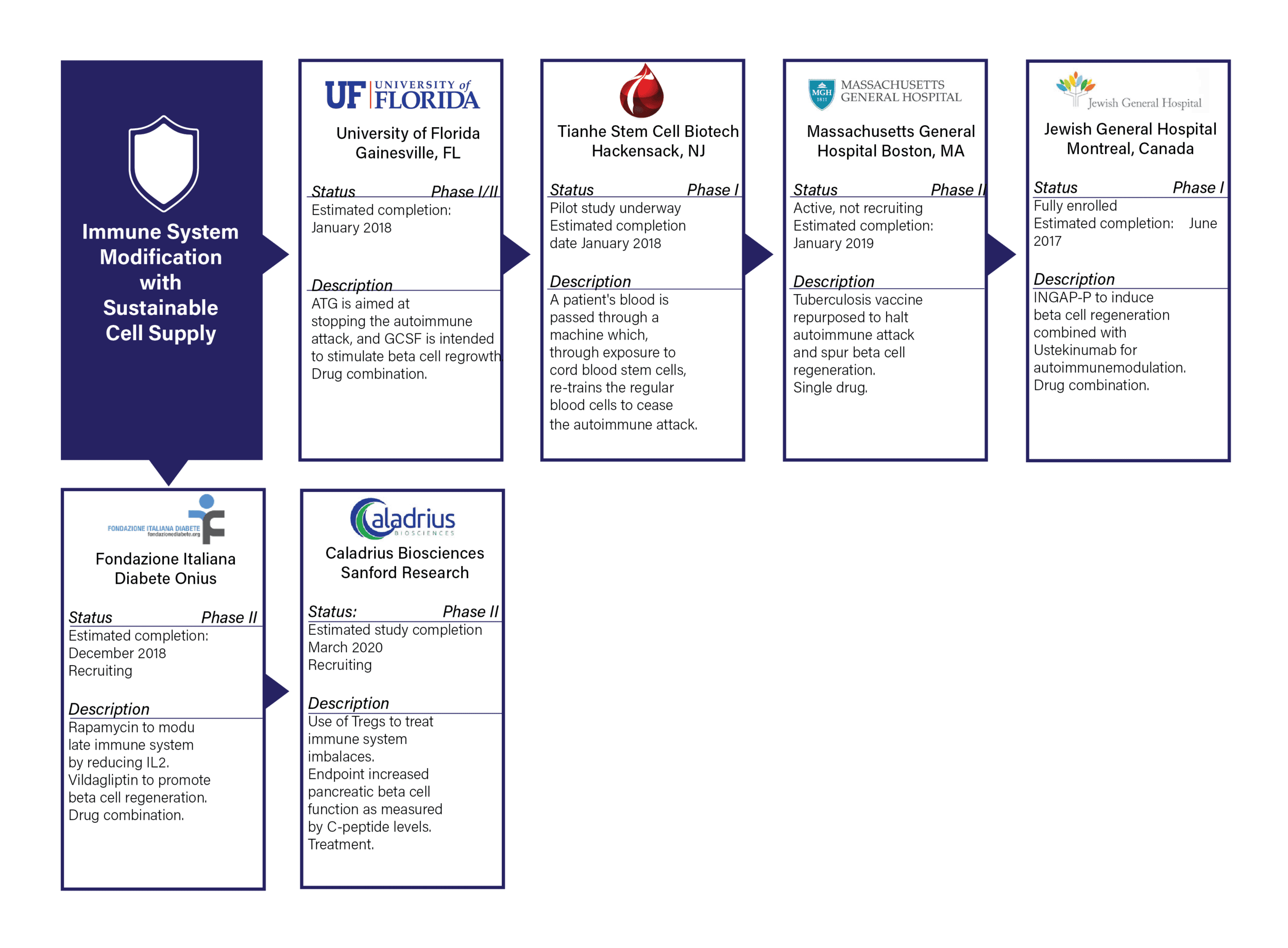
- Definition: Therapy to stop the immune system from destroying beta cells, including modifying, blocking, and retraining.
- Commentary: This pathway has the largest number of active trials and has seen an increase in active trials over the last five years. One concern within this pathway is immune system modification research is often targeted at T1D prevention, as opposed to a cure to those currently living with the disease.
- There are six active trials, all of which are ongoing.
GLUCOSE RESPONSIVE INSULIN & THE ARTIFICIAL PANCREAS
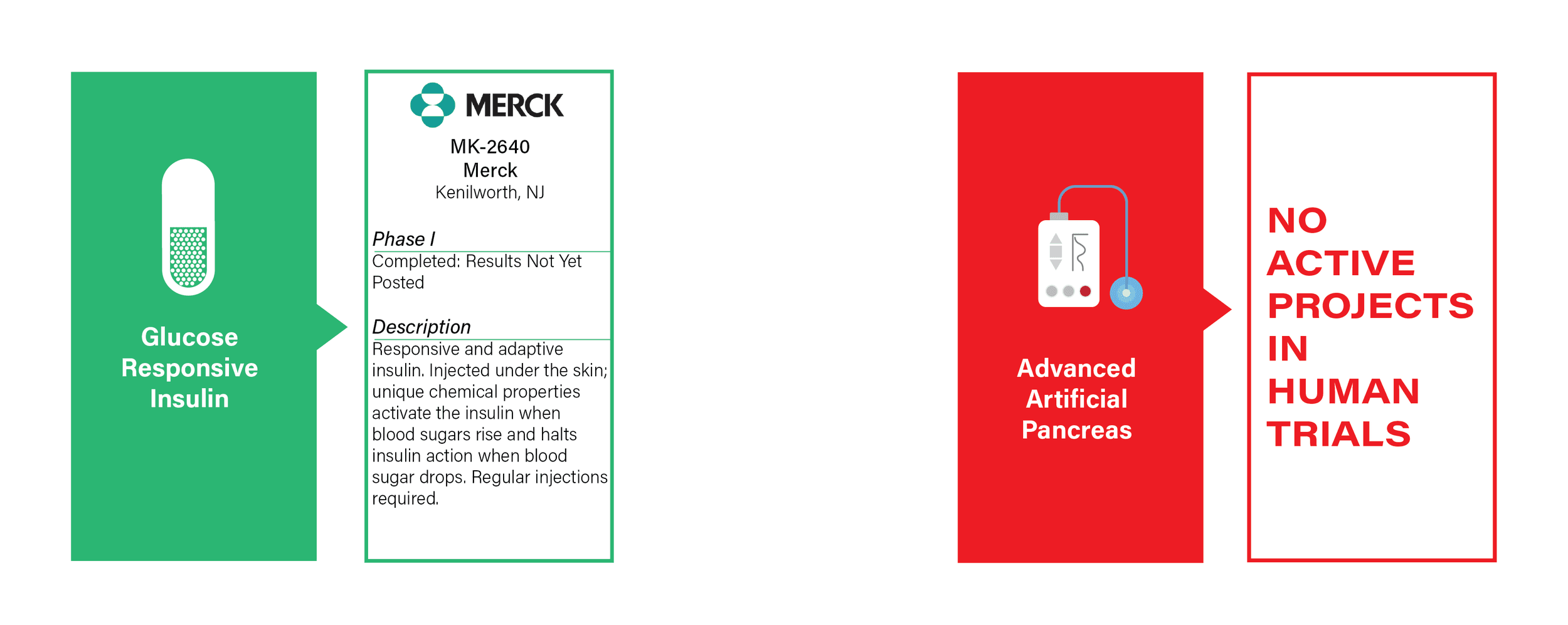
- Smart Insulin Definition: “Smart insulin” is injected and chemically activates only in response to changes in blood sugar.
- Commentary: This pathway has seen advances over the past few years but Merck remains the only entity active in clinical trials. A number of other smart insulin approaches are being pursued, including smart insulin patches, intranasal insulin, as well as the traditional pill form.
- One trial is completed but has not yet posted results.
- Artificial Pancreas Definition: A device that mimics the pancreas by monitoring changes in blood sugar and independently administers insulin without the patient’s input.
- Commentary: To be considered a Practical Cure the device must be small enough for users to completely forget about its presence. No current devices are small enough.
- There are no active Artificial Pancreas Practical Cure projects.
APPENDIX A: EMERGING PRACTICAL CURE PROJECTS
Emerging PC projects include projects which may begin human trials in the next two years. The projects listed below have received a significant amount of media coverage and represent the next-in-line PC projects. However, because none of these projects are currently testing in humans, they are likely more than 15 years away from FDA approval.
CELL TRANSPLANT WITH AUTOIMMUNE PROTECTION
- Pharmacyte Biotech has begun testing its Cell-in-a-Box microencapsulation technology. Its insulin-producing Melligen cells, derived from liver cells, are being tested in mice. There is no current timeline for moving into clinical trials.
- Orgenisis has developed a process to convert a patient’s liver cells into insulin-producing cells. Two clinical trials are scheduled to be performed in Germany and Belgium starting in 2017.
- The DRI transplanted islet cells into the omentum in one human patient in an ongoing proof of concept phase I/II study.
- Sernova is developing an encapsulation “pouch” the size of a credit card and containing donor islets. The company terminated a Phase I/II trial earlier this year.
- Semma Therapeutics has commercialized Doug Melton’s work using stem cells to create beta cells. If successful, this work could be a key component of a Practical Cure. The project remains more than 24 months from starting human trials.
IMMUNE SYSTEM MODIFICATION
- DiaVacs is working on a reverse vaccine to stop the autoimmune attack. The company plans to initiate clinical studies in 2018.
- Imcyse is developing an immune system modification therapy which employs modified peptides to alter T cell behavior. The company has an 18 site trial starting sometime in 2017.
GLUCOSE RESPONSIVE INSULIN
- Sanofi is working on smart insulin solutions in collaboration with MIT, UNC, and others. There is no current timeline for moving into clinical trials.
ARTIFICIAL PANCREAS PROJECTS
- Currently no emerging PC projects.
