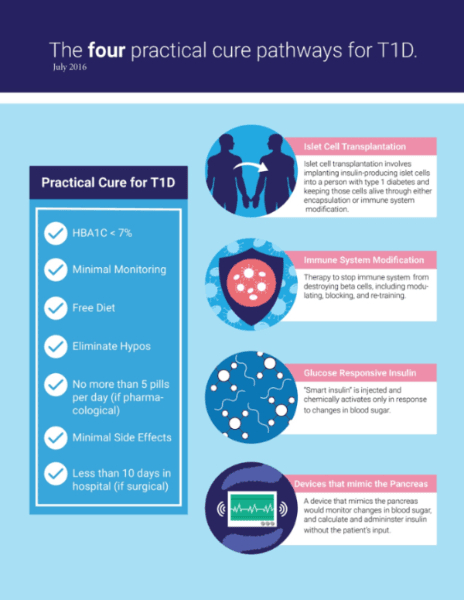
This is the fourth annual review of type 1 diabetes human clinical trial research currently underway. The primary objective of the review is to identify the projects in human trials that may result in a Practical Cure for T1D. The main conclusion from this review is that there continue to be very few projects in human trials which have a chance to bring to market a Practical Cure. See "Appendix A" for an info-graphic defining a Practical Cure for T1D.
All FDA approved clinical trials are registered in clinicaltrials.gov and are publicly available. We included in this analysis all projects that were a) open but not yet recruiting b) actively recruiting c) active but no longer recruiting new participants.
KEY SUMMARY POINTS:
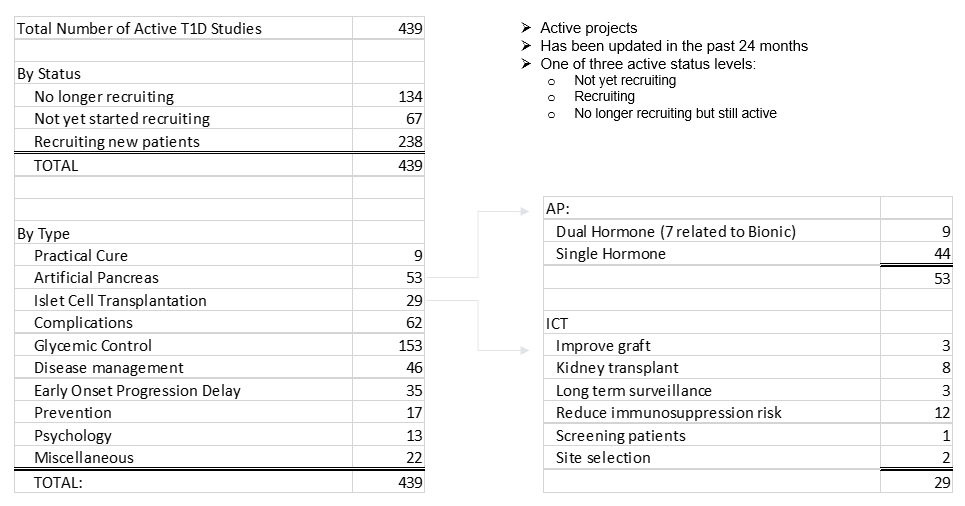
- 439 type 1 diabetes projects are currently underway in FDA-sanctioned human clinical trials.
- 9 projects have the potential to be a practical cure, less than 2% of the total.
- 153 projects address glycemic control (not including artificial pancreas work). This is the largest concentration of projects.
- 53 projects are addressing the artificial pancreas, a number that has been increasing over recent years. 9 of these projects are dual hormone with both insulin and glucagon, while 44 are insulin only.
See "Appendix B" for a one-page summary of key numbers.
PRACTICAL CURE PROJECTS IN HUMAN TRIALS BY PATHWAY:
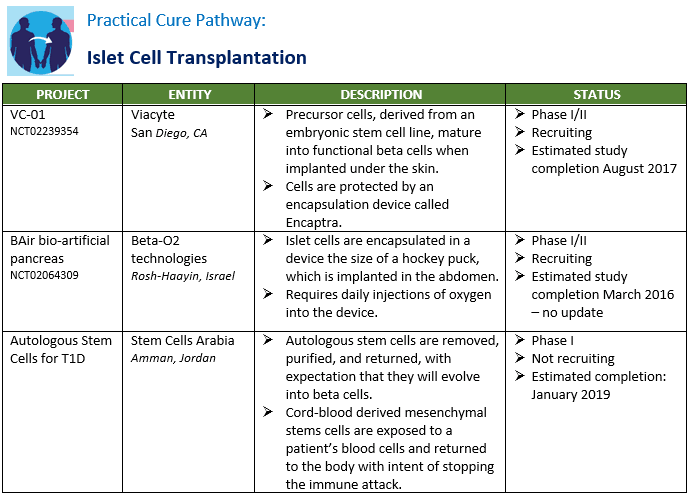
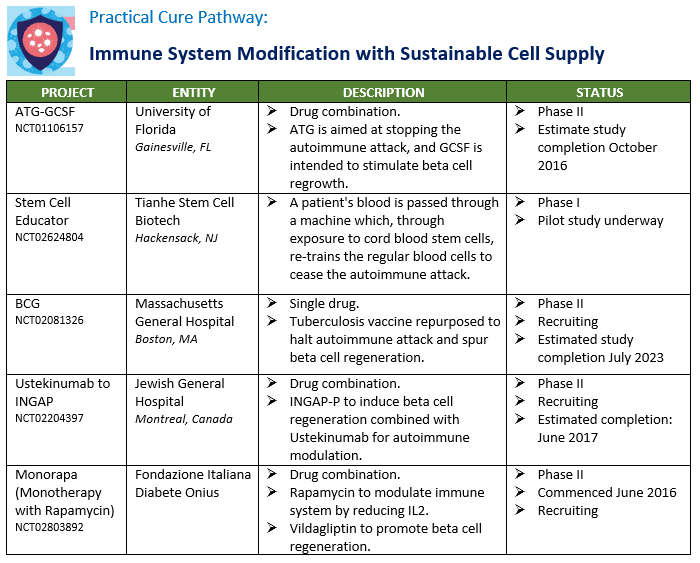
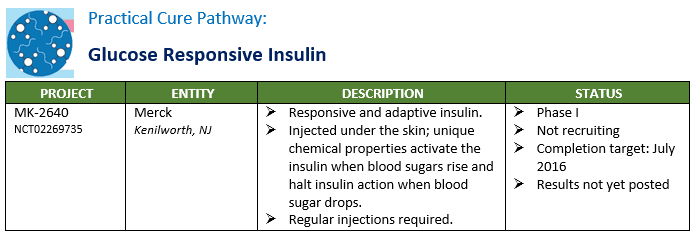
There are nine active Practical Cure research projects currently underway in human clinical trials. A summary of these projects is presented in the charts below by pathway. The FDA NCT number is included in the chart for anyone who wishes to read the full filing registered in clinicaltrials.gov.
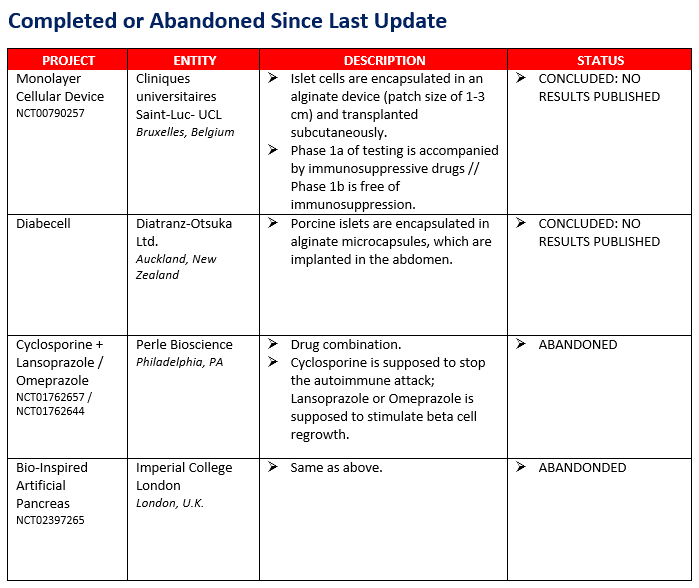
There have been several changes since the last update. Two projects concluded but no results have yet been published and two other projects were prematurely terminated. See "Appendix C" for a list of completed and abandoned. At the same time, three new projects have entered the charts, one being conducted in Amman, Jordan, one in Montreal, and one in Italy. The Amman study seeks to stop the autoimmune attack by training stem cells and is similar to the work being conducted at Hackensack Medical Center by Tianhe, which has been on the list for a few years. The Montreal study tests a drug combination that revisits work conducted in the previous decades with INGAP, a protein that encourages beta cell regeneration, and combines it with an autoimmune modulation agent. Finally, the Italy study is testing a drug combination intended to spur beta cell growth and simultaneously halt the autoimmune attack.
The most substantive change from the November 2015 list is the treatment of device pathway. As noted in the Pathways report published last week, the JDCA conducted preliminary survey research to determine the criteria that the T1D community would require to consider them a Practical Cure. In order for any device to meet the criteria to be considered a Practical Cure it must be small enough for users to completely forget about its presence. While there continues to be tremendous enthusiasm and support for the current generation of AP devices being tested, none of these are currently minimal enough.

APPENDIX A:
APPENDIX B:
APPENDIX C: