New Phase III study results on islet cell transplantation were published on April 18. This report will compare the results from this wave of research, conducted between 2008 and 2014, and the previous wave of research, conducted between 2001 and 2007.
BASIC DEFINITION OF ISLET CELL TRANSPLANTATION:
In its simplest version, islet transplantation is a procedure that combines both islet cell supply and ongoing cell protection. The approach usually has three main parts:
- Securing a source of fully functioning, insulin-producing cells. Today, the only proven source of islets is recently deceased humans and, accordingly, both sets of research results reflect studies that sourced beta cells from cadavers.
- Inserting those cells into the body through a simple surgical procedure. In theory, the transplant procedure should improve with experience.
- Keeping the transplanted cells alive with some form of protection, such as immune suppression or encapsulation. To date, the only proven way to suspend the autoimmune attack is by taking immune-suppressing medication, which reduces the body’s overall disease-fighting capability. Both sets of research results make use of immune-suppression drugs which must be taken for life.
REAL-LIFE REASON FOR HOPE:
While islet cell transplantation in its current iteration is far from a Practical Cure to T1D, it has delivered extraordinary results in a handful of real people. The DRI, a clear leader in this area of work, have treated three individuals who underwent this procedure and have been insulin independent for over 10 years.
That said, because cell supply is so limited and full-body immune suppression is still perceived to carry more long-term risk than current T1D insulin use, it has a long way to go before it can be utilized as a Practical Cure for the majority of people with T1D. However, because of the proven success in humans to date, albeit a small number, it does point to specific and focused challenges to solve and, in our view, merits continued support from the organizations that are working towards these ends.
BACKGROUND ON THE TWO SETS OF RESULTS:
Following the Edmonton Protocol established in 2000, a group of 32 research centers joined forces to broadly implement the protocol and track results under the guidance of the U.S. National Institute of Diabetes and Digestive and Kidney Disease. The group published annual reports of research results, the last of which was released in 2011. This final report was cumulative of all research to date.
Over the study time frame, many of the 32 research centers dropped out. At the end of the period, only a handful of centers were still actively conducting and testing these procedures. The remaining centers set up a new joint effort called the Clinical Islet Transplantation Consortium (CITC) to continue the work forward. The April 18 publication reveals the CITC's first public research results.
A quick comparison of the main design characteristics is listed in the chart below:

RESULTS COMPARISON:
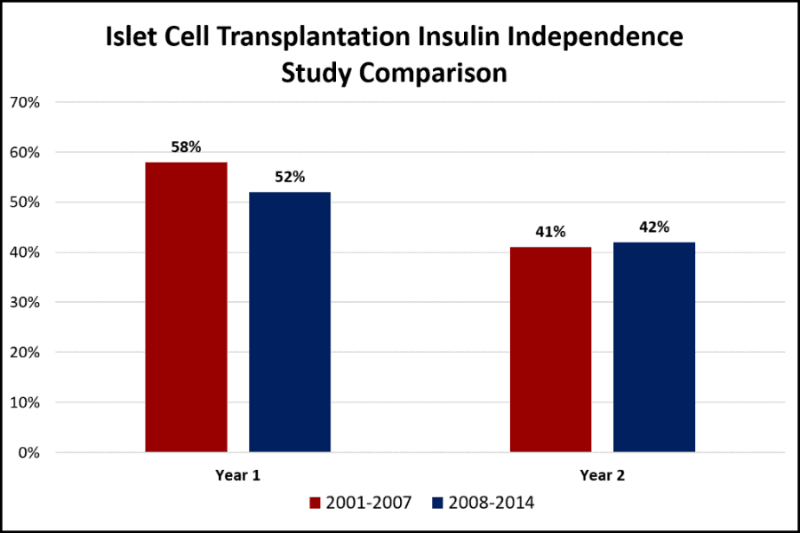
In our view, the most important factor that points toward a cure is the number of procedure recipients who remain insulin independent with normal HBA1C levels. Additional factors recorded are adverse events (i.e., major issues related to the procedure or immune suppression drugs) and reduction of severe hypoglycemic events. Summary results are compared in the following chart; a more comprehensive comparison can be found in the appendix.
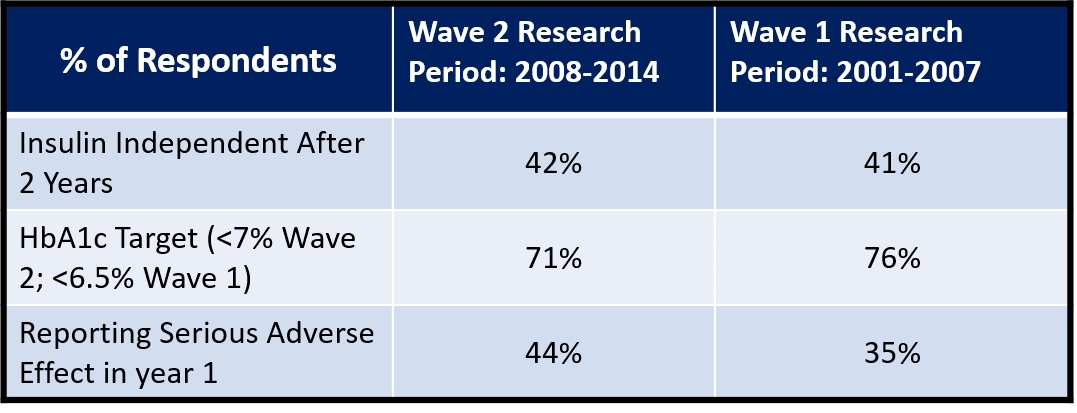
On net, the results are very similar to the prior wave which suggests ongoing relevance and value in moving forward with this pathway of research. One key takeaway is the limited number of recipients in the Wave 2 study. At only 48, the pool is perhaps too small to result in material advances. A concentrated and focused clinical effort that combines the latest advances in cell supply and cell protection seems like a no-brainer and the best chance for future success.
RELATED LINKS:
- 2001 to 2007 Data: https://web.emmes.com/study/isl/reports/01062012_7thAnnualReport.pdf
- 2008 to 2014 Data: http://care.diabetesjournals.org/content/early/2016/04/12/dc15-1988.full.pdf
- JDCA Report: http://thejdca.org/islet-transplanation-overview
- CITR Website: http://www.citregistry.org/
- CITC Website: http://www.citisletstudy.org/
- CITC Study: http://care.diabetesjournals.org/content/early/2016/04/12/dc15-1988.full.pdf
