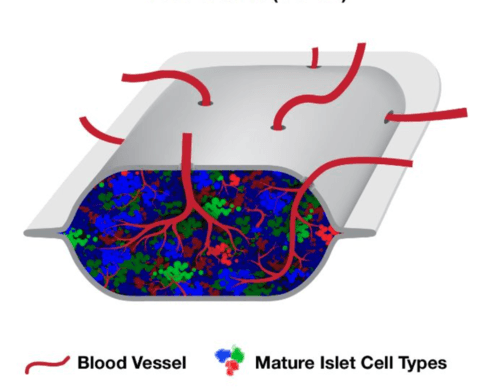
Photo: Schematic representation of PEC-Direct (VC-02), which is comprised of pancreatic progenitor cells in a pouch-type device designed to allow blood vessels to enter through perforations in the membrane. Image courtesy of ViaCyte, Inc.
One of the most talked-about companies pursuing a T1D Practical Cure is ViaCyte, the San Diego company that hopes to use stem cells to provide a functional cure for T1D. The company has raised over $100 million, mostly from the California Institute of Regenerative Medicine (CIRM), which is itself funded by California State bonds.
In 2021, the company’s stem cell-derived islets were shown, in a peer-reviewed journal, to produce insulin in people with T1D. These results were described as “proof of concept” that surgical implantation of stem-derived cells can replace the function of the pancreas. This is certainly exciting news, but how close are we, really, to a cure for T1D? Are the latest results truly a research breakthrough?
This report will summarize the status of ViaCyte’s research. The report is based, in part, on an interview exchange in December of 2021 with ViaCyte researchers and representatives.
Core Product: PEC Cells
ViaCyte’s core product is its “pancreatic endoderm cells” (“PECs”). PEC cells begin as pluripotent stem cells with the potential to develop into almost any kind of cells. They are grown in a lab to become “pancreatic progenitors,” which mature into insulin-producing cells once implanted in the body.
To function in people, PEC cells need to be protected from the T1D autoimmune attack and the body’s natural reaction to a foreign entity. ViaCyte is currently testing several different ways to keep the cells healthy with three product candidates. PEC-Direct is the “first-generation” product that pairs PEC cells with full-body immunosuppressive drugs; this product will only be available for a small percentage of people with T1D. Additional iterations, called PEC-Encap and PEC-QT, aim to eliminate the need for immunosuppression so that they can be available to everyone with T1D.
PEC-Direct
What it is: PEC cells are transplanted within an open scaffold that creates an environment to grow into insulin-producing cells. People who receive this surgery must take full-body immune-suppressing drugs to avoid immune rejection of the transplanted cells.
Status:
- The results published in 2021 were the first-ever evidence reported in a journal of stem-derived cells producing insulin in people with T1D.
- The patient who experienced the greatest clinical benefit saw their external insulin use reduced by 69% in the year following implantation.
- None of the 17 people in the trial became insulin-independent at any point.
- Two patients dropped out of the study because the marginal clinical benefit they received did not merit the continued use of immune-suppressing drugs
- ViaCyte is continuing to recruit patients for this trial and plans to share more data in 2022.
- ViaCyte Commentary: When we asked ViaCyte if people with T1D should feel good about the latest PEC-Direct results, they said, “Yes. We can now definitively say that stem cell-derived islet cells can survive and function long-term and safely in patients with type 1 diabetes.”
Further, the company is optimistic that, with further iterations, the product can be evolved to achieve insulin independence.
JDCA Perspective: In our view, this is indeed exciting news that may, down the road, provide a more plentiful supply of insulin-producing cells than is currently available. However, we are still in the early days of testing. The trial is very small – we have only seen data from 17 people – and no one achieved insulin independence. Further, this product requires full-body immunosuppressants and is therefore not a Practical Cure.
PEC-Encap
What it is: PEC cells are implanted within an “encapsulation” device that is designed to prevent immune cells from directly contacting the implanted cells, so they may thrive and function without provoking an immune response or being destroyed.
Status:
- The PEC-Encap trial that was re-started at the beginning of last year closed once again in 2021 because the cells did not function optimally within the device.
- The company plans to take what it learned from this study and start a new trial of encapsulated islets in 2022.
ViaCyte Commentary: When asked why the encapsulation device failed, ViaCyte was tight-lipped, stating that this is competitively sensitive information. But the company did say that it will continue its partnership with W.L. Gore, the company that manufactures Goretex, to create a membrane that “achieves a balance between effective delivery and cell engraftment with the therapeutic function of implanted cells.”
JDCA Perspective: While it is disappointing news that the latest attempt at an encapsulation solution did not work, it is good that ViaCyte is committed to taking the learning and continuing to iterate. ViaCyte has the resources to push the envelope of encapsulation, which has flummoxed researchers for years—a breakthrough here would certainly be a big step towards a Practical Cure.
PEC-QT
What it is: PEC cells genetically engineered using CRISPR Cas-9 technology to be invisible to the immune system. They are implanted within an open-scaffold device.
Status:
- The PEC-QT cells evaded autoimmune detection in mice.
- As we reported last week, the first patient was dosed in a clinical trial in which ten people will be implanted with the device.
- The outcomes of this trial are all related to safety endpoints. Insulin production is not a primary or secondary endpoint of the trial.
- This will be the first CRISPR Cas-9 product ever been tested in people with T1D.
ViaCyte Commentary: When asked about the status of the trial, the company said that it has “already begun the process to screen and enroll patients in the near term,” but was “unable to comment on specific timelines at this time.”
JDCA Perspective: CRISPR Cas-9 is at the forefront of gene-editing technology, and it’s worth noting that one of the scientists who won a Nobel Prize for pioneering the technology founded the company that ViaCyte is partnering with on this effort. We are excited to see the results of the first human testing of CRISPR Cas-9 technology in people with T1D. However, until there are results in people, it is not worthwhile to speculate on the efficacy of this product.
Final Thoughts: A Race is On
It is important to note that ViaCyte is not the only horse in this race. On the other coast of the United States, another company also made headlines in 2021 with promising results and similar, stem-cell-based, capabilities.
Vertex Pharmaceuticals, the Boston-area company best known for its life-changing billion-dollar Cystic Fibrosis drug portfolio, has become a major player in type one diabetes. Last fall, the company released early results from a human trial in a press release that showed one participant (on full-body immunosuppression) reduced his need for external insulin by 90% three months into the trial.
Vertex purchased Semma Therapeutics in 2018, a company founded by Harvard Professor Doug Melton, another pioneer of stem cell technology for T1D. Unlike ViaCyte’s cells, Vertex’s cells are fully-functioning upon implantation; they produce insulin in a test tube before they are even transplanted into the body.
Vertex and ViaCyte are now the front runners in the race to supply insulin-producing cells for T1D. As far as we can tell, the companies are racing the track neck-to-neck. In the best-case scenario, this competition will spur us to a T1D Practical Cure sooner rather than later.
