This report provides an overview of all smart insulin projects, also known as glucose-responsive insulin. Smart insulin is one of the four JDCA Practical Cure pathways, but it is important to note that all smart insulin products in development are in pre-clinical testing.
Key Takeaways
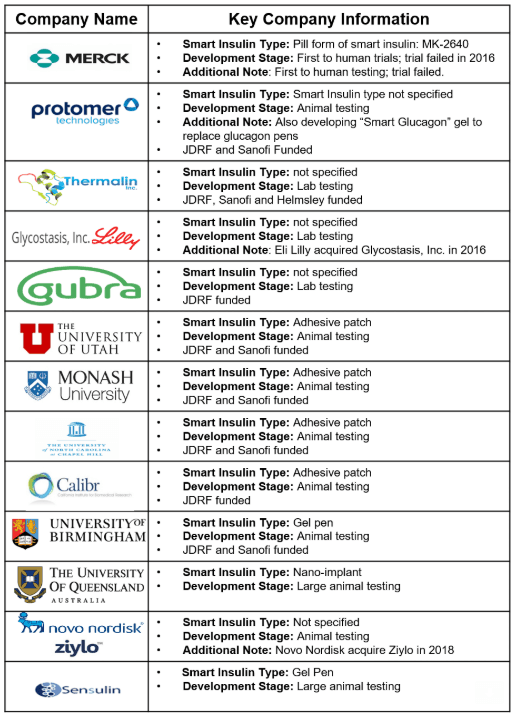
- 12 smart insulin projects are in development: six at commercial entities and six at different universities.
- There are four different types of smart insulin being tested. See infographic below.
- Merck is the only company to advance to human trials. The trial failed, and the company is currently trying to advance a backup candidate.
- JDRF has funded eight of the 12 projects across 23 grants ($9.3 million).
- Several pharmaceutical companies have invested in smart insulin over the last decade including Eli Lilly, Novo Nordisk, and Sanofi.
- Device materials, the glucose-sensing ability and insulin response, and protection from the immune system all present problems and continue to be studied.
Definition
Smart insulin is chemically activated in response to changes in blood glucose levels. It remains inactive inside the body until blood glucose rises above normal levels. At that point, the chemical component activates the insulin response. Once blood glucose returns to normal, the insulin action ceases, avoiding low blood sugar. In theory, smart insulin could potentially decrease the amount of regular blood-glucose tests required and dramatically reduce the number of daily injections to one per day.
As noted above, there are four main types of smart insulin in development:

History and Overview
The development of smart insulin first started in the late 70s, but early prototypes failed due to the body’s immune response. Throughout the 80s and 90s, scientists continued to develop smart insulin models with a focus on device material and capabilities, without any major breakthroughs. However, in 2010, Merck, one of the largest pharmaceutical companies in the world, acquired a small startup called SmartCells which had developed a smart insulin pill. In 2014, Merck began the first smart insulin phase-I human trial— a major step forward. Unfortunately, in 2016 the Merck trial was terminated for lack of efficacy.
Despite Merck's setback, over the last few years, a number of notable companies, universities, and major T1D non-profits (including JDRF) have begun to invest in smart insulin projects. To date, there is no timeline for when these other smart insulin products will begin testing in humans.
While smart insulin has seen advances over the past few decades, two main issues continue to present development obstacles, as noted above: (1) The glucose-sensing ability and insulin response. In order to be safe, smart insulin needs to release the correct amount of insulin in response to changes in glucose in the body. Solving this issue necessitates a bio-material which can hold and contain insulin for long stretches of time, respond to glucose changes, release precise doses when needed, and shut off when blood sugar levels are normalized. (2) Protection from the body’s immune response. Early models of smart insulin failed because they were attacked as a foreign object once inside the body. In order to be successful, any smart insulin product would need to remain in the body without being attacked.
The Smart Insulin Path to a Practical Cure:
To qualify as a Practical Cure, smart insulin would have to last long enough to eliminate the need for multiple daily injections. Although no current smart insulin product has been able to achieve this, the projects in this analysis are only the first wave of products being tested.As technology and medical advancements are often rapidly iterative, the JDCA remains hopeful that a smart insulin project will make its way through the testing pipeline and into human trials in the near future.
Appendix A: Competitor Details