This is the first of two reports which will address Practical Cure projects currently in human trials. This report defines a Practical Cure and identifies the four most likely research pathways. The second report will provide an updated list of projects by pathway that are currently in human trials.
PRACTICAL CURE IS OUTCOME FOCUSED
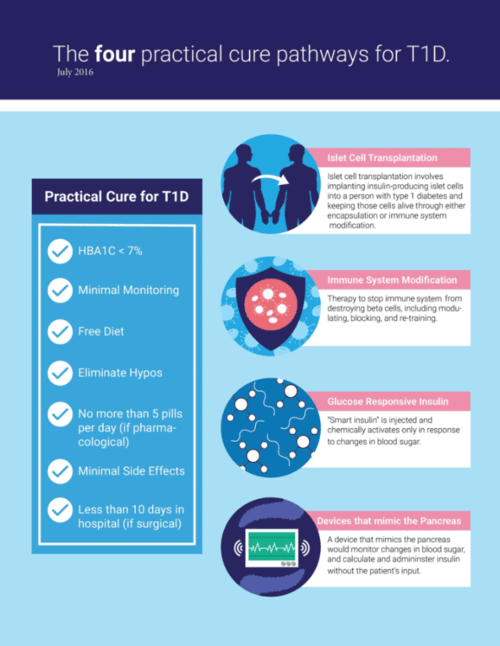
A Practical Cure is any solution which minimizes the disruptive aspects of T1D and enables a near-normal quality of life; it is purely outcome focused. The opening chart shows the various outcome criteria that a Practical Cure must meet, including sleeping worry-free, no dietary restrictions, minimal monitoring, insignificant side effects, elimination of hypos, and HbA1C readings under 7%.
In addition, any Practical Cure solution must have a reasonable chance of being available within the next fifteen years — in time to transform the lives of people who are currently living with the disease. Considering that, on average, it requires 10-15 years from the beginning of human trials to receive FDA pre-market approval, research projects that are currently human clinical trials have the best chance of meeting the timetable.
THE PATHWAYS
1) Islet Cell Transplantation involves implanting insulin-producing islet cells into a person with type 1 diabetes. It has three major components:
- Cell protection: The islet cells must be protected from the immune attack after they have been implanted in the body. Various encapsulation approaches have been tested in humans. Immune-suppressing drugs are another alternative, but side effects still have to be reduced.
- Cell supply: The only proven source of Islet cells is cadavers, which have very limited availability. Research into deriving a sustainable cell supply from human stem cells has seen promising advances and is currently being tested in two different human trials.
- Site selection: Islet cells require large supplies of oxygen and nutrients in order to survive. The current protocol is to transplant islet cells in the liver, where most of them die. Other sites, including the stomach lining and the area under the skin, are being tested as alternatives.
2) Immune System Modification stops the body’s immune system from attacking insulin-producing beta cells, either through drugs or stem cell therapy. Currently, human trials are testing the utility of regenerating beta cells alongside immunotherapy in type 1 diabetics with the goal of producing sufficient amounts of insulin. If regeneration proves ineffective, blocking the autoimmune attack would need to be combined with islet cell transplantation. There are currently four active projects in human trials.
3) Glucose-Responsive Insulin, aka “smart insulin,” is chemically activated in response to changes in blood glucose. Once injected, smart insulin remains inactive until blood glucose rises above normal levels. At that point, the chemical component activates the insulin, and once blood glucose returns to normal, the insulin action ceases, avoiding low blood sugar. To qualify as a Practical Cure, smart insulin would have to last long enough to eliminate the need for multiple daily injections. There is currently one active project in human trials.
4) A Device that Mimics the Pancreas, often referred to as an artificial pancreas, is under development at several commercial and academic centers. The JDCA recently completed a survey asking the T1D community to identify the requirements an artificial pancreas must meet in order to qualify as a Practical Cure. The main factors were reliability, effectiveness at controlling blood sugar, and size. 88% of respondents said an AP Device would be a Practical Cure if "it is small enough that you could generally forget that you are wearing it." There are no active projects in human trials because no current generation AP projects are small enough.
